Abstract
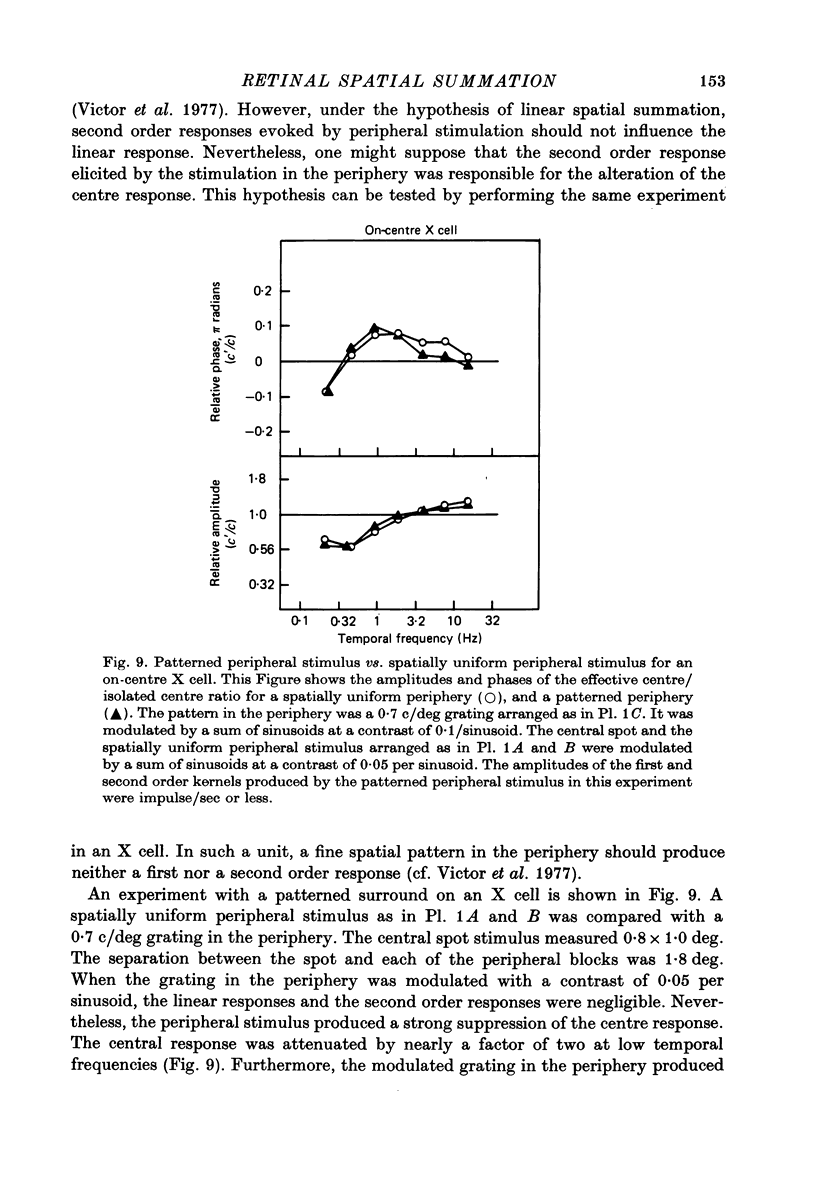
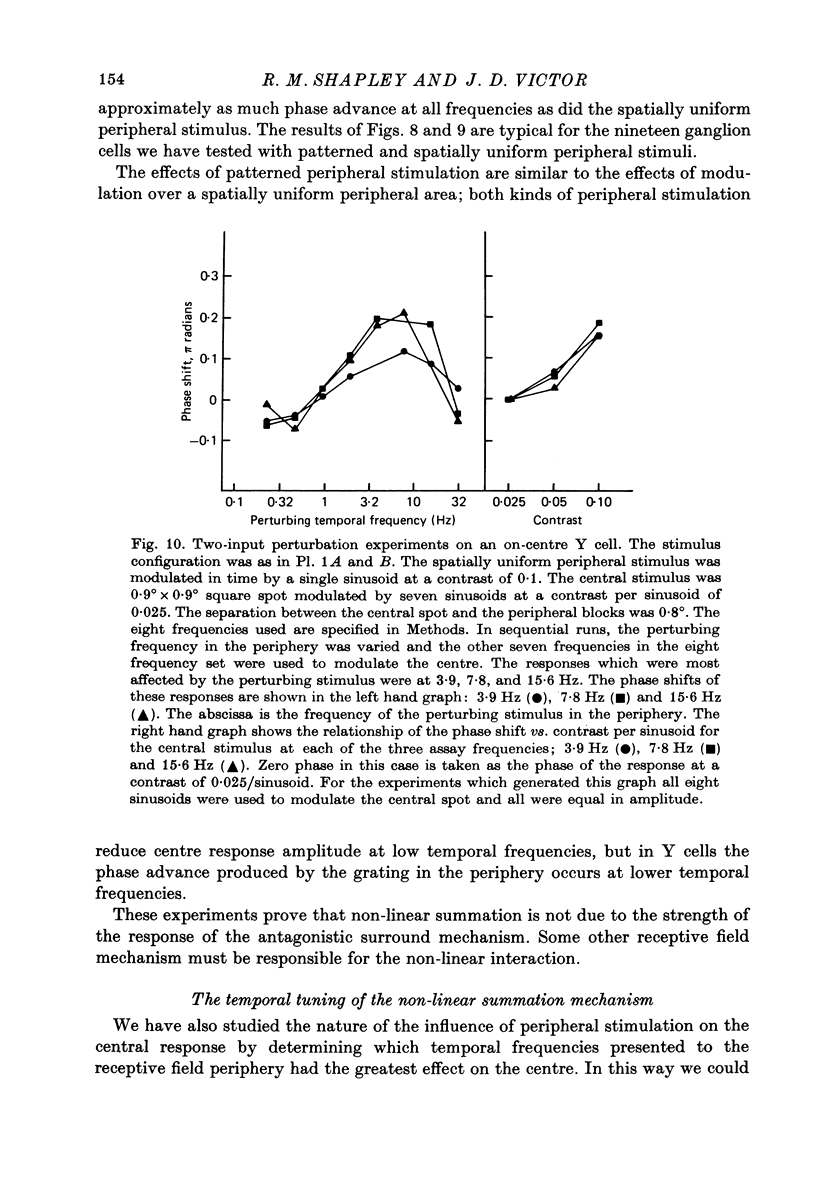
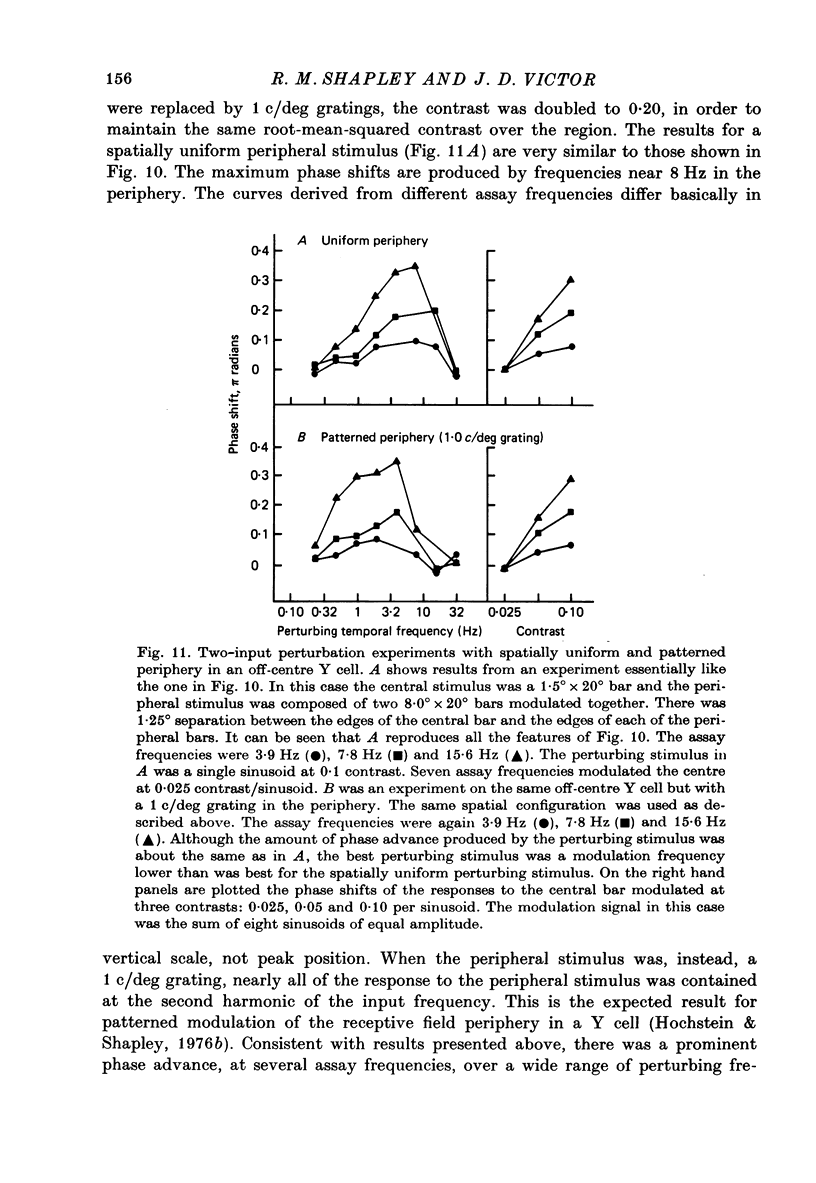
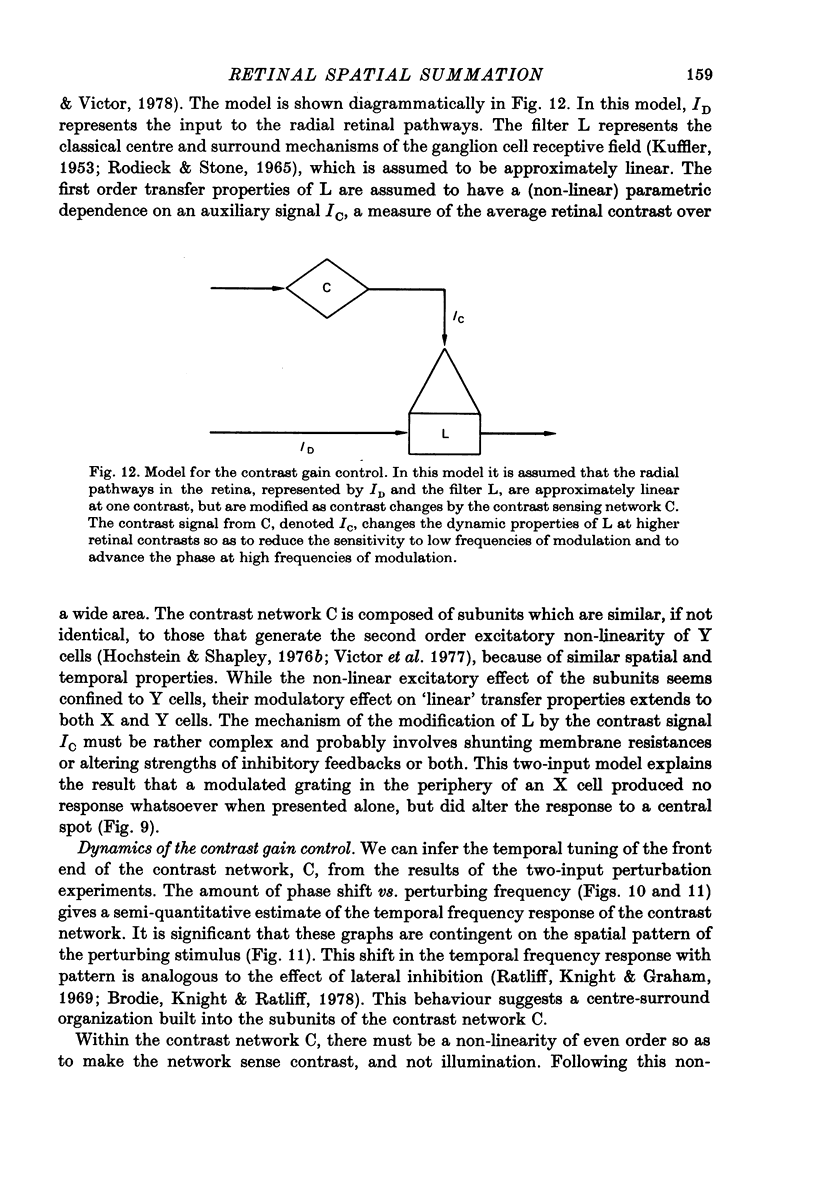
1. We studied how responses to visual stimuli at spatially separated locations were combined by cat retinal ganglion cells. 2. The temporal signal which modulated the stimuli was a sum of sinusoids. Fourier analysis of the ganglion cell impulse train yielded first order responses at the modulation frequencies, and second order responses at sums and differences of the input frequencies. 3. Spatial stimuli were spots in the centre and periphery of the cell's receptive field. Four conditions of stimulation were used: centre alone, periphery alone, centre and periphery in phase, centre and periphery out of phase. 4. The effective first order response of the centre was defined as the response due to centre stimulation in the presence of periphery stimulation, but independent of the relative phases of the two regions. Likewise, the effective first order response of the periphery was defined as the response due to periphery in the presence of centre stimulation, but independent of the relative phases of the two regions. These effective responses may be calculated by addition and subtraction of the measured responses to the combined stimuli. 5. There was a consistent difference between the first order frequency kernal of the effective centre and the first order kernel of the centre alone. The amplitudes of the effective centre responses were diminished at low frequencies of modulation compared to the isolated centre responses. Also, the phase of the effective centre's response to high frequencies was advanced. Such non-linear interaction occurred in all ganglion cells, X or Y, but the effects were larger in Y cells. 6. In addition to spatially uniform stimuli in the periphery, spatial grating patterns were also used. These peripheral gratings affected the first order kernal of the centre even though the peripheral gratings produced no first order responses by themselves. 7. The temporal properties of the non-linear interaction of centre and periphery were probed by modulation in the periphery with single sinusoids. The most effective temporal frequencies for producing non-linear summation were: (a) 4-15 Hz when all the visual stimuli were spatially uniform, (b) 2-8 Hz when spatial grating patterns were used in the periphery. 8. The characteristics of non-linear spatial summation observed in these experiments are explained by the properties of the contrast gain control mechanism which we have previously postulated.
Full text
PDF



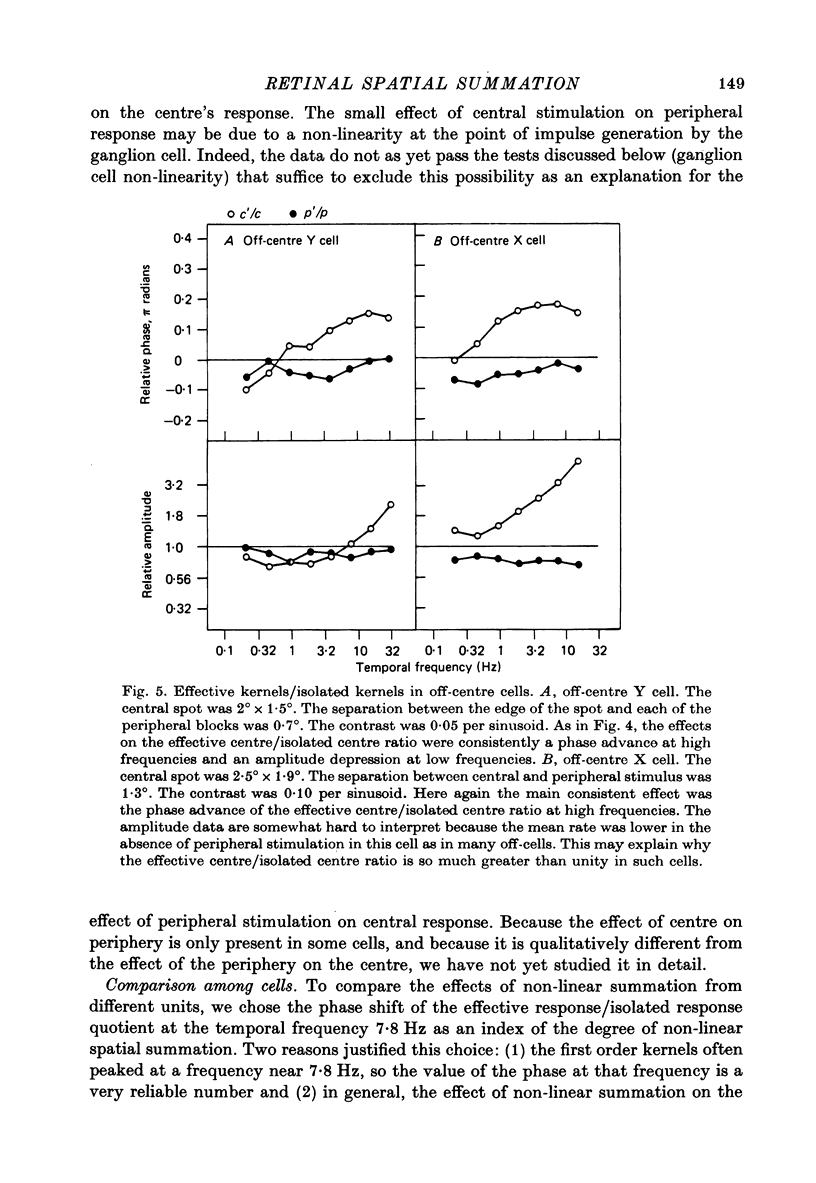
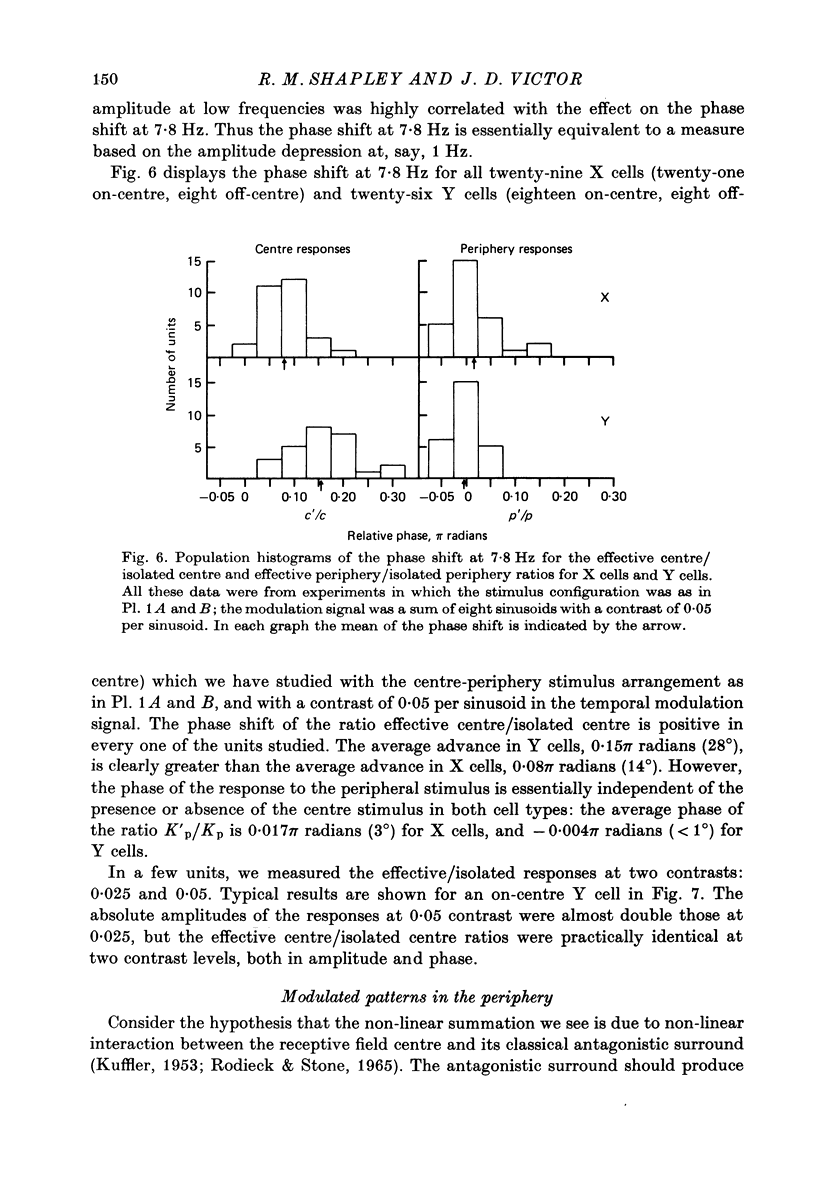
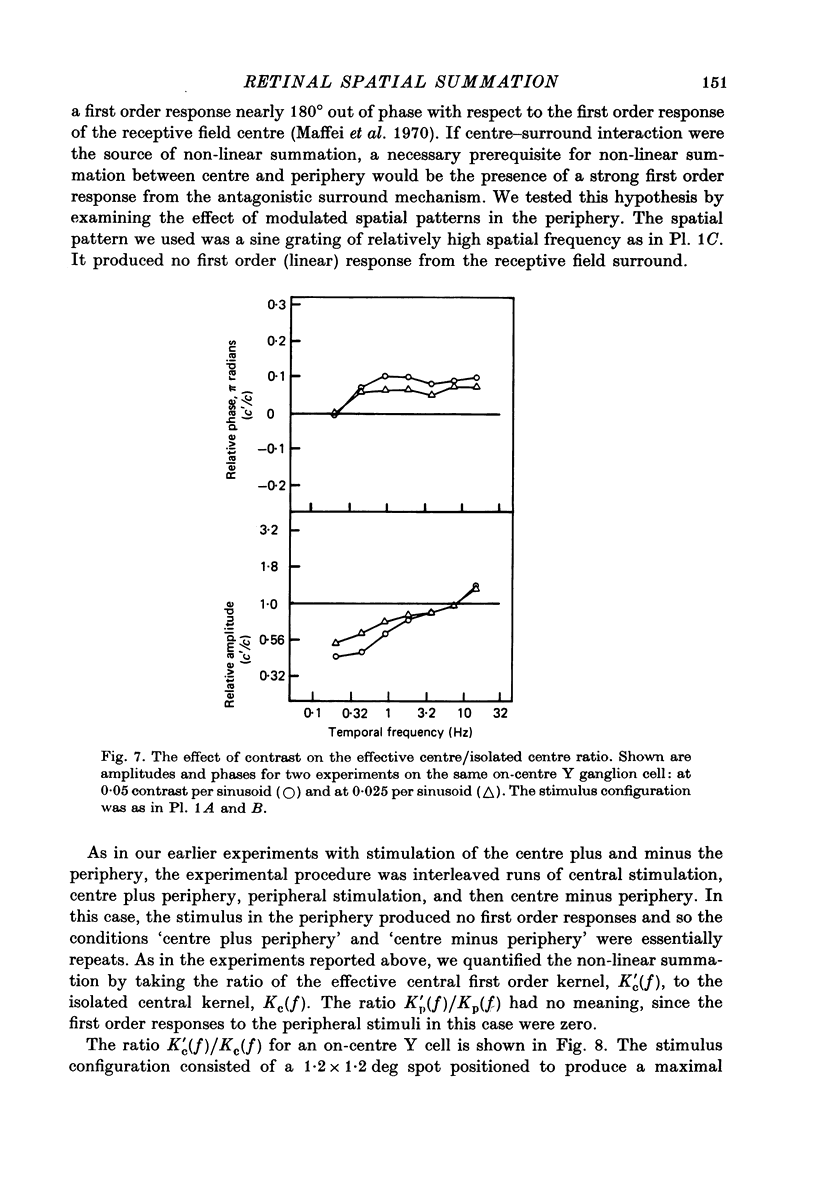
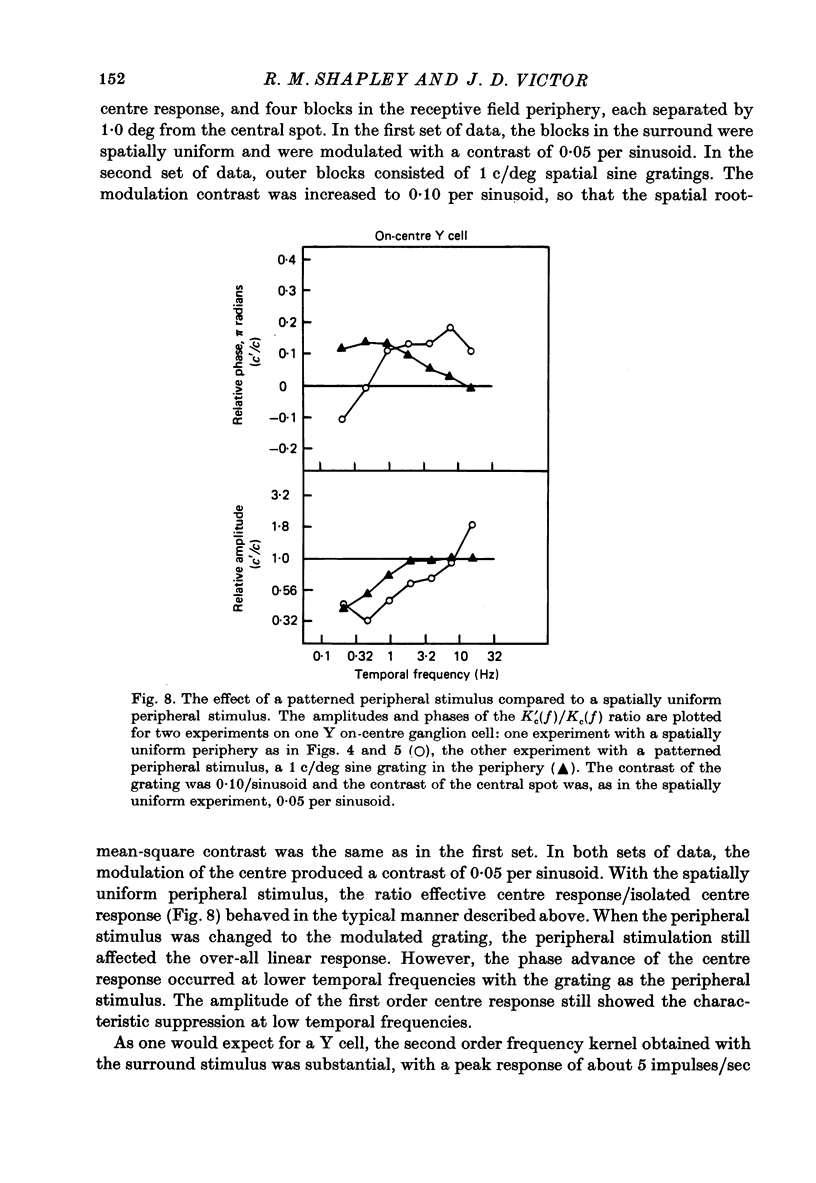





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie S. E., Knight B. W., Ratliff F. The spatiotemporal transfer function of the Limulus lateral eye. J Gen Physiol. 1978 Aug;72(2):167–202. doi: 10.1085/jgp.72.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. Algebraic summation of centre and surround inputs to retinal ganglion cells of the cat. Nature. 1970 May 2;226(5244):458–459. doi: 10.1038/226458a0. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L. Dynamic interactions in retinal receptive fields. Vision Res. 1968 Oct;8(10):1299–1303. doi: 10.1016/0042-6989(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- Ratliff F., Knight B. W., Graham N. On tuning and amplification by lateral inhibition. Proc Natl Acad Sci U S A. 1969 Mar;62(3):733–740. doi: 10.1073/pnas.62.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978 Dec;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M., Knight B. W. Nonlinear analysis of cat retinal ganglion cells in the frequency domain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3068–3072. doi: 10.1073/pnas.74.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]



