Abstract
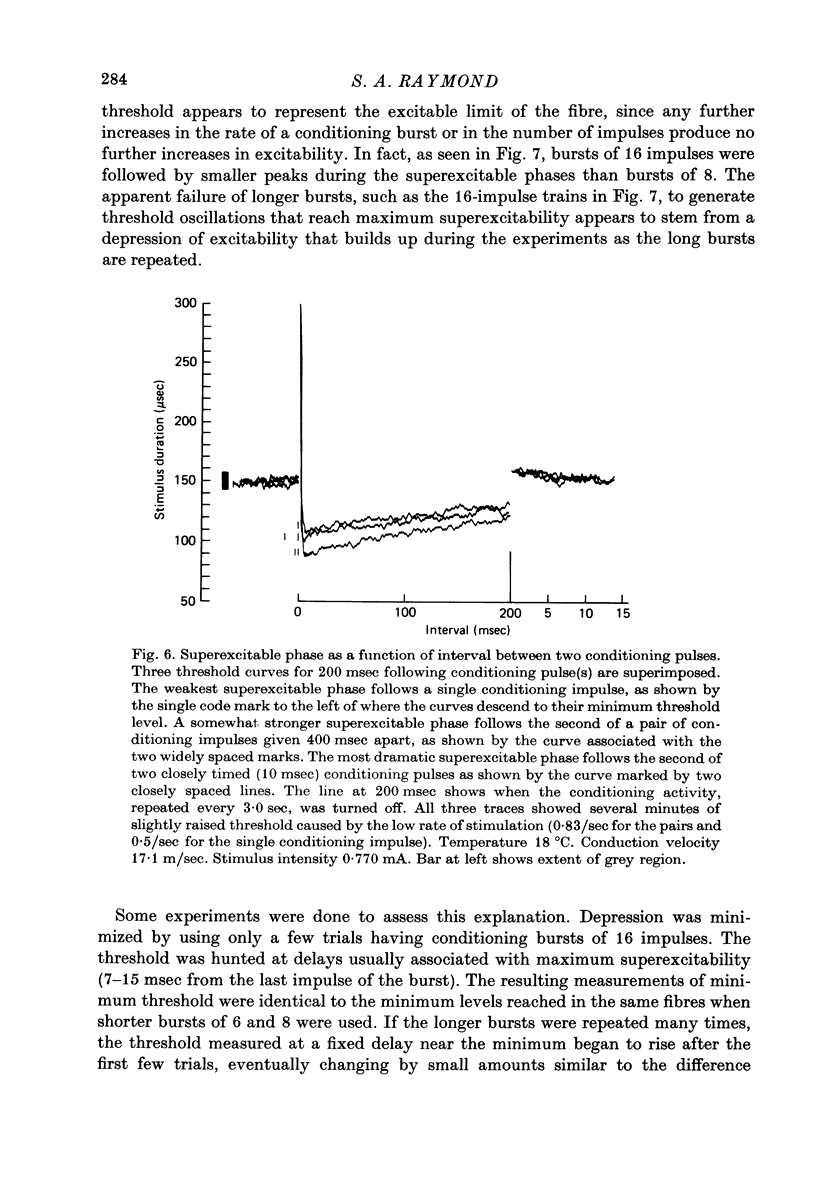
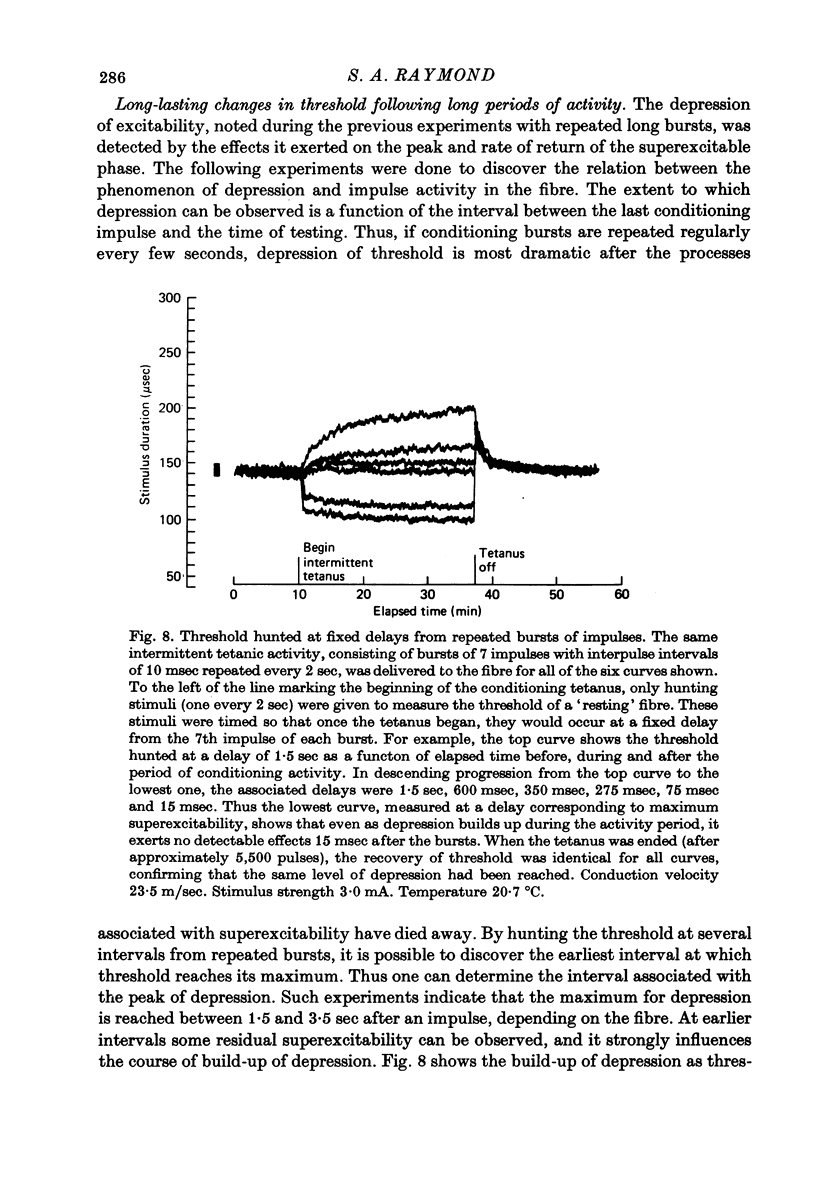
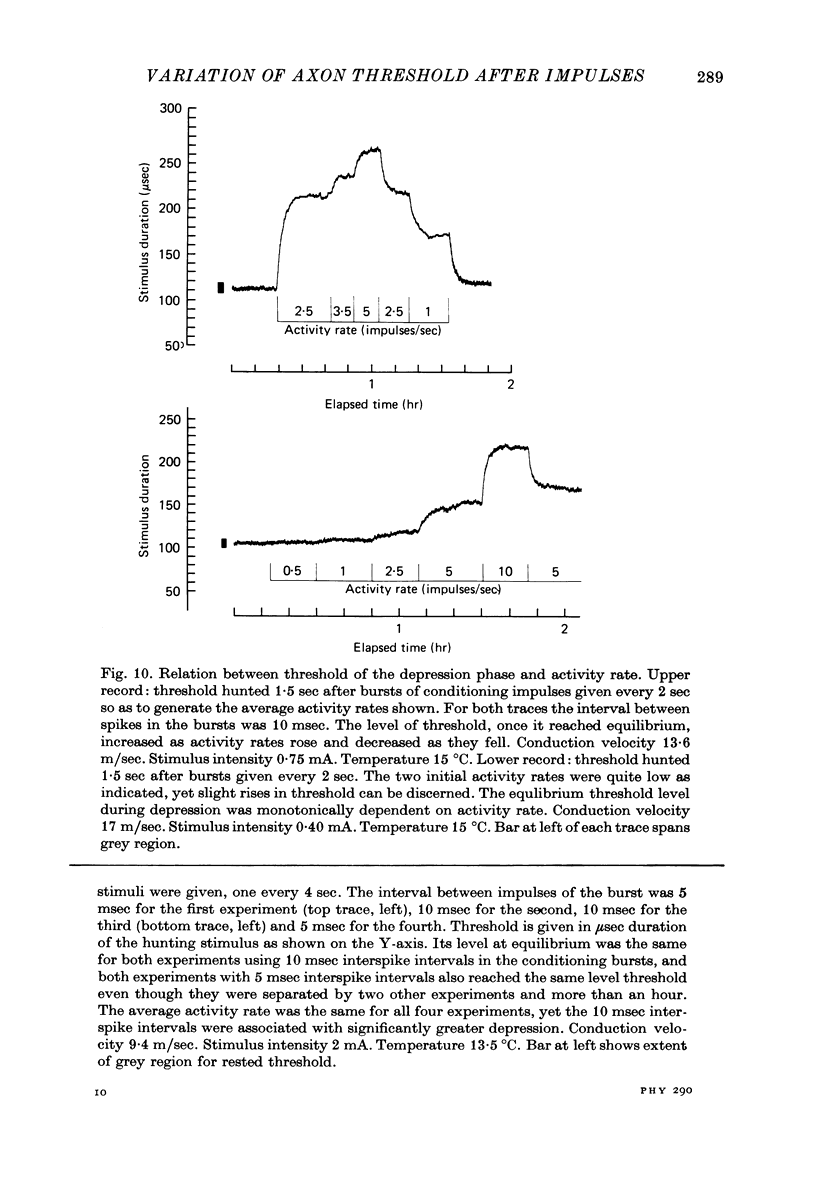
1. The firing thresholds of single myelinated fibres of frog sciatic nerves were monitored as a function of impulse activity in the fibre. The threshold was given by the number of coulombs in current pulses that excited a particular fibre half the time when delivered to the whole nerve. Threshold was tracked by a device that incrementally decreased the number of coulombs in the current pulse whenever the fibre responded and increased the pulse if it did not respond. 2. There was a pattern to the after-oscillations of threshold following activity. The fibres were briefly refractory, transiently superexcitable for about 1-1.5 sec and then entered a phase of raised threshold or 'depression' that lasted for many minutes. 3. Activity produced little change in the threshold curve during the refractory period. Strong depressions following prolonged activity prevented the threshold from returning to the base-line level within the time associated with the refractory period for the same fibre at rest. 4. After an impulse, superexcitability reached a maximum within 7-20 msec. This peak was larger as the number of impulses in a preceding burst increased and as the intervals between the impulses became briefer. Each successive impulse of a burst contributed less to the growth of superexcitability, and after the burst had 6-10 impulses additional impulses contributed nothing. 5. The depression phase was marked by the interaction between build-up, which depended on the activity rate, and recovery, which required as long as an hour or more for the threshold to be completely restored to resting level. These two mechanisms, one causing build-up and the other recovery, led to formation of dynamic equilibria. The threshold level at equilibrium increased monotonically with the activity rate. 6. The processes associated with superexcitability interact with those producing depression. In active fibres showing raised thresholds, impulses are followed by a relative superexcitability that persists for at least as long as an absolute superexcitability (with threshold below the resting level) can be measured in the same fibre at rest. 7. The duration of the superexcitable phase interpreted as a relative change in excitability was roughly the same regardless of the level of depression. 8. The magnitude of the oscillation in threshold was give to ten times larger than the grey region (the range of stimuli for which response is probabilistic). It is concluded that at regions of low conduction safety such as axonal branches, where weak forces can influence whether an impulse will pass, such pronounced and long-lasting after-effects of firing can be expected to modulate conduction of nerve impulses.
Full text
PDF



























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. The recovery process of excitable tissues: Part II. J Physiol. 1921 Aug 3;55(3-4):193–225. doi: 10.1113/jphysiol.1921.sp001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHTHAL F., ENGBAEK L. REFRACTORY PERIOD AND CONDUCTION VELOCITY OF THE STRIATED MUSCLE FIBRE. Acta Physiol Scand. 1963 Nov;59:199–220. doi: 10.1111/j.1748-1716.1963.tb02737.x. [DOI] [PubMed] [Google Scholar]
- BULLOCK T. H. Facilitation of conduction rate in nerve fibers. J Physiol. 1951 Jun;114(1-2):89–97. doi: 10.1113/jphysiol.1951.sp004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Raymond S. A., Lettvin J. Y. Multiple meaning in single visual units. Brain Behav Evol. 1970;3(1):72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- DUN F. T. The delay and blockage of sensory impulses in the dorsal root ganglion. J Physiol. 1955 Feb 28;127(2):252–264. doi: 10.1113/jphysiol.1955.sp005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUN F. T. The terminal arborization of nerve fibres as an important factor in synaptic and neuromuscular transmission. J Cell Physiol. 1951 Aug;38(1):133–135. doi: 10.1002/jcp.1030380111. [DOI] [PubMed] [Google Scholar]
- Decima E. E., Goldberg L. J. Centrifugal dorsal root discharges induced by motoneurone activation. J Physiol. 1970 Mar;207(1):103–118. doi: 10.1113/jphysiol.1970.sp009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLIATT R. W., WILLISON R. G. The refractory and supernormal periods of the human median nerve. J Neurol Neurosurg Psychiatry. 1963 Apr;26:136–147. doi: 10.1136/jnnp.26.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. An extreme supernormal period in cerebellar parallel fibres. J Physiol. 1972 Apr;222(2):357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. A. Changes in interspike interval during propagation: quantitative description. Biol Cybern. 1977 Jun 13;26(4):209–213. doi: 10.1007/BF00366592. [DOI] [PubMed] [Google Scholar]
- Goldstein S. S., Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J. 1974 Oct;14(10):731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLAND B., LETTVIN J. Y., McCULLOCH W. S., PITTS W., WALL P. D. Reflex inhibition by dorsal root interaction. J Neurophysiol. 1955 Jan;18(1):1–17. doi: 10.1152/jn.1955.18.1.1. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P. C., McINTYRE A. K. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke's column. J Neurophysiol. 1950 Jan;13(1):39–54. doi: 10.1152/jn.1950.13.1.39. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH W. S., LETTVIN J. Y., PITTS W. H., DELL P. C. An electrical hypothesis of central inhibition and facilitation. Res Publ Assoc Res Nerv Ment Dis. 1952;30:87–97. [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Parnas I., Hochstein S., Parnas H. Theoretical analysis of parameters leading to frequency modulation along an inhomogeneous axon. J Neurophysiol. 1976 Jul;39(4):909–923. doi: 10.1152/jn.1976.39.4.909. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A. 1977 Jan;74(1):211–215. doi: 10.1073/pnas.74.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O., Hatt H. Axon conduction block in a region of dense connective tissue in crayfish. J Neurophysiol. 1976 Jul;39(4):794–801. doi: 10.1152/jn.1976.39.4.794. [DOI] [PubMed] [Google Scholar]
- Spear J. F., Moore E. N. Supernormal excitability and conduction in the His-Purkinje system of the dog. Circ Res. 1974 Nov;35(5):782–792. doi: 10.1161/01.res.35.5.782. [DOI] [PubMed] [Google Scholar]
- Spira M. E., Yarom Y., Parnas I. Modulation of spike frequency by regions of special axonal geometry and by synaptic inputs. J Neurophysiol. 1976 Jul;39(4):882–899. doi: 10.1152/jn.1976.39.4.882. [DOI] [PubMed] [Google Scholar]
- Swadlow H. A., Waxman S. G. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol. 1976 Oct;53(1):128–150. doi: 10.1016/0014-4886(76)90288-0. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973 May;230(3):509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verveen A. A., Derksen H. E. Amplitude distribution of axon membrane noise voltage. Acta Physiol Pharmacol Neerl. 1969 Aug;15(3):353–379. [PubMed] [Google Scholar]
- Waxman S. G., Pappas G. D., Bennett M. V. Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Stern archus. J Cell Biol. 1972 Apr;53(1):210–224. doi: 10.1083/jcb.53.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G., Swadlow H. A. Morphology and physiology of visual callosal axons: evidence for a supernormal period in central myelinated axons. Brain Res. 1976 Aug 20;113(1):179–187. doi: 10.1016/0006-8993(76)90017-2. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976 Dec;263(3):513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]


