Abstract
1. The effect of catecholamines on cyclic adenosine 3′5′-monophosphate (cyclic AMP) production in isolated rat superior cervical ganglia has been measured under experimental conditions in which they also produce ganglion hyperpolarization.
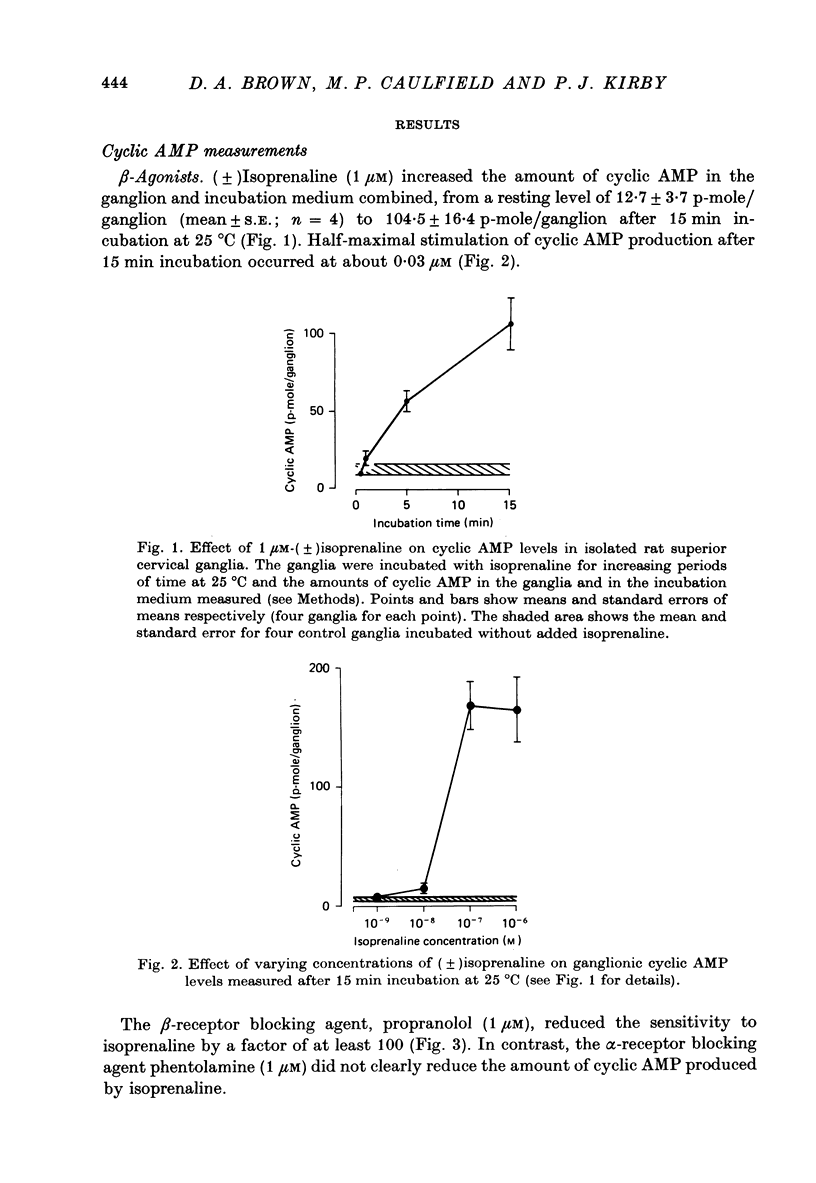
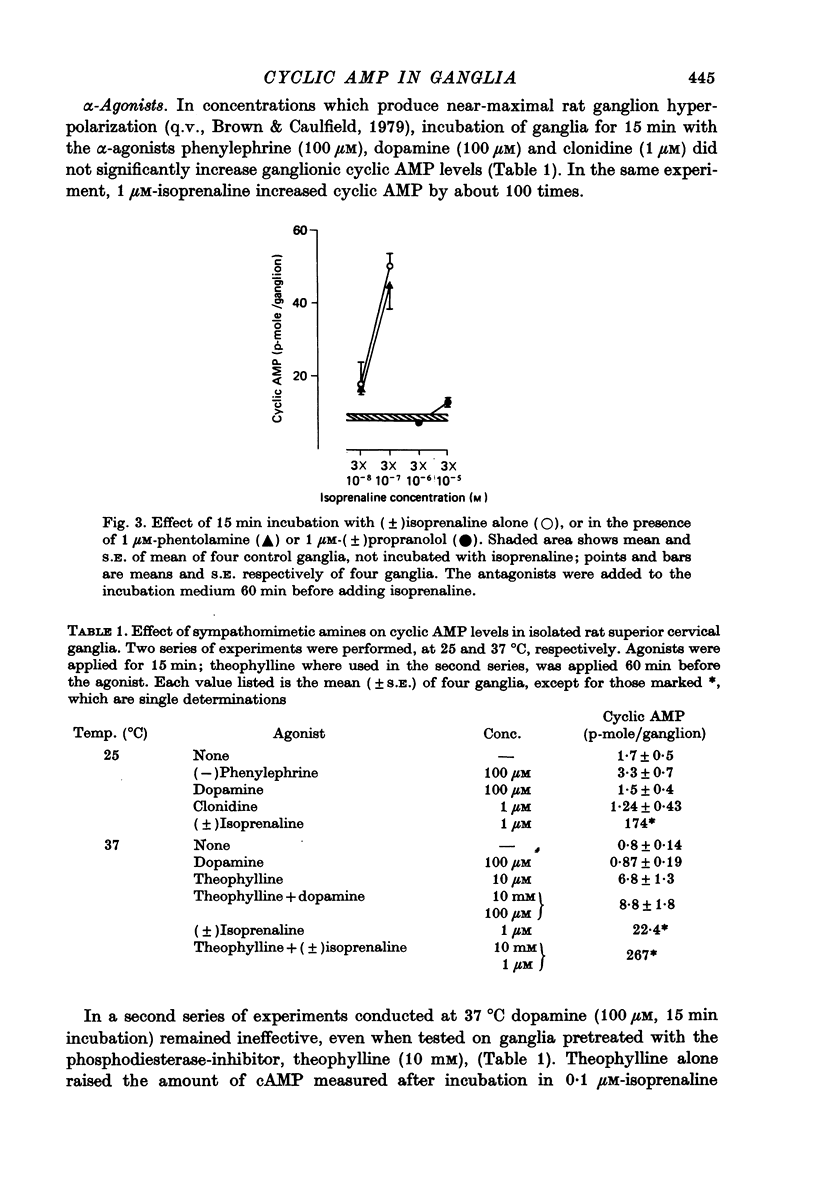
2. (±)Isoprenaline (1 μM) increased cyclic AMP levels by 8-100 times after 15 min incubation at 25 °C. Half-maximal stimulation occurred at about 0.03 μM. This was due to stimulation of β-receptors, since it was prevented by 1 μM-propranolol but not by 1 μM-phentolamine.
3. The α-agonists phenylephrine (100 μM), dopamine (100 μM) and clonidine (1 μM) did not produce a detectable increase in ganglionic cyclic AMP. Dopamine (100 μM) was also ineffective at 37 °C in the presence of 10 mM-theophylline.
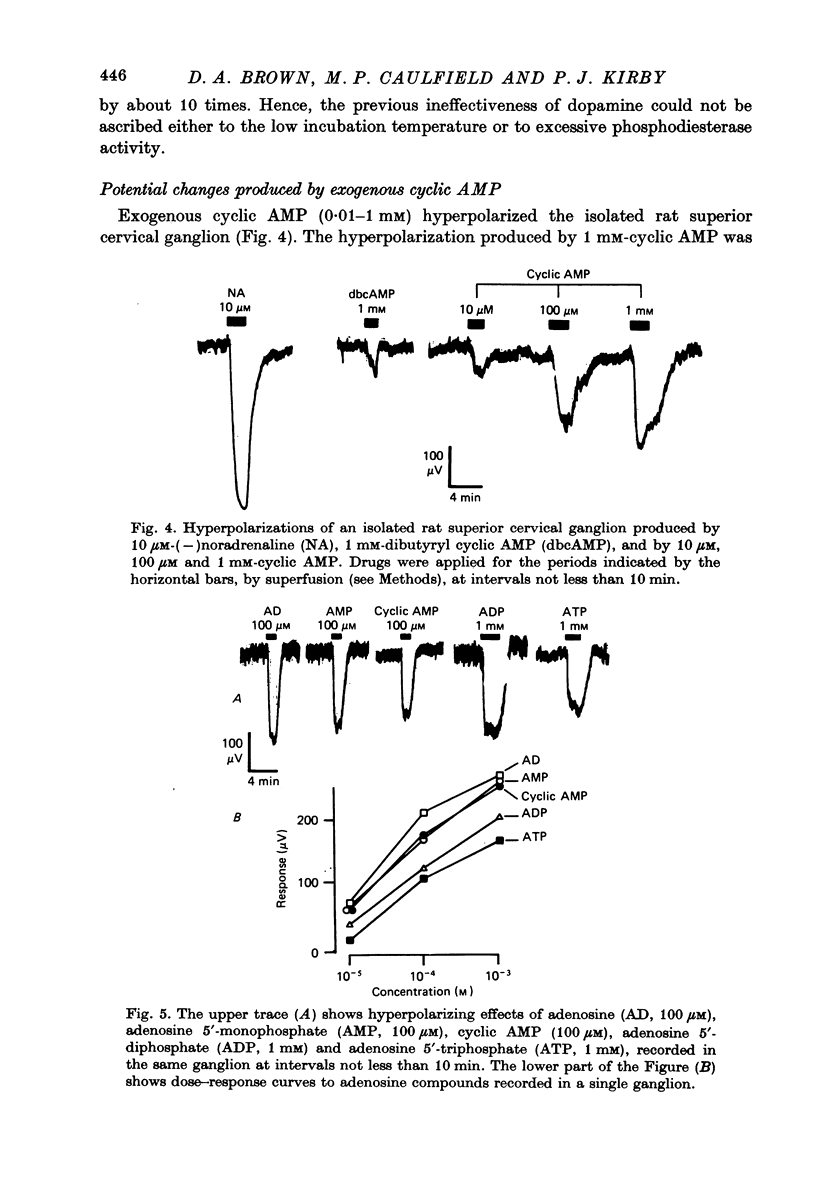
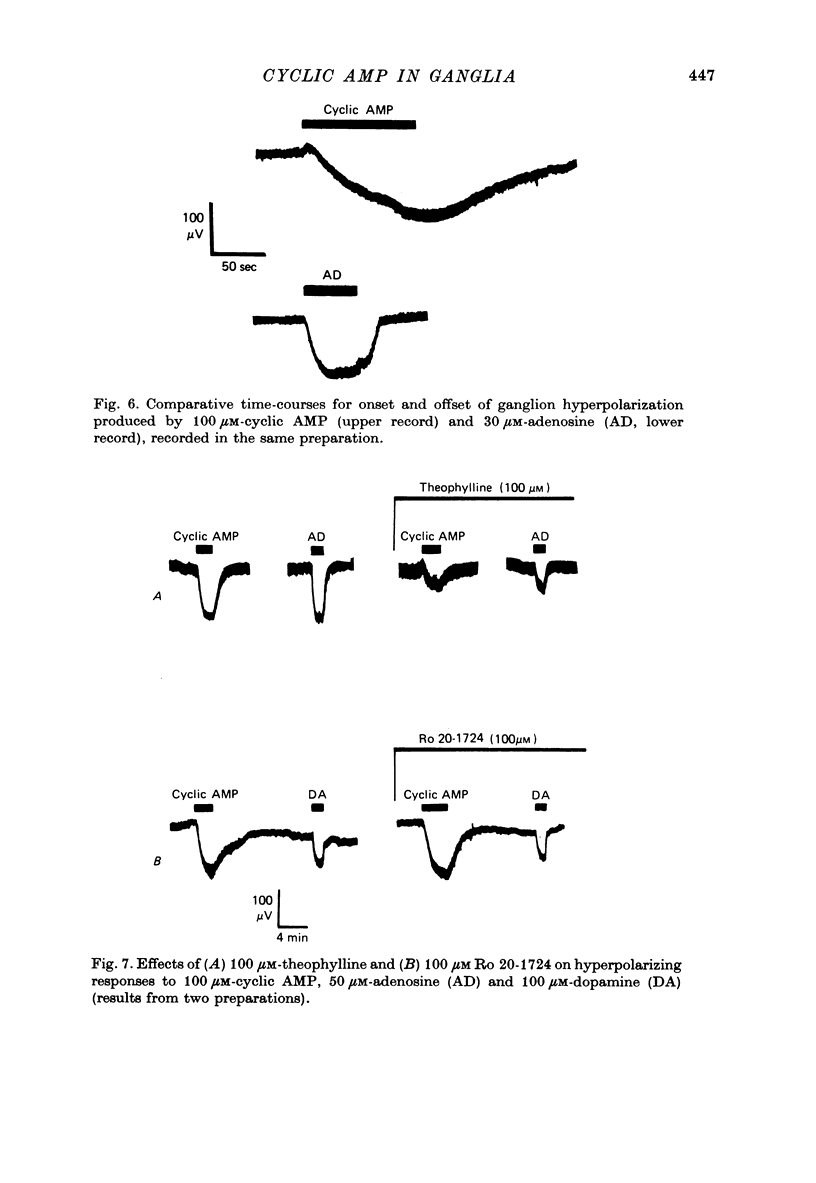
4. Exogenous cyclic AMP (0.01-1 mM) hyperpolarized the ganglion. This effect was replicated by other adenosine compounds, most effectively by adenosine and by adenosine 5′-monophosphate, and was antagonized by theophylline. Dibutyryl cyclic AMP was weaker than cyclic AMP.
5. Neither theophylline nor the non-xanthine phosphodiesterase inhibitor, Ro 20-1724, enhanced the hyperpolarizing actions of noradrenaline or dopamine.
6. Since catecholamine-induced hyperpolarization of the isolated rat ganglion is induced via α-receptors, whereas cyclic AMP-production is induced via β-receptors, it is concluded that cyclic AMP is unlikely to mediate the hyperpolarization. The effect of exogenous cyclic AMP may be due to an action on external adenosine-receptors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Koketsu K. Effects of dibutyryl cyclic adenosine 3',5'-monophosphate and theophylline on the bullfrog sympathetic ganglion cells. Br J Pharmacol. 1977 Jul;60(3):331–336. doi: 10.1111/j.1476-5381.1977.tb07504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. A very simple method for recording ganglion depolarization. J Physiol. 1975 Mar;246(2):24P–26P. [PubMed] [Google Scholar]
- Busis N. A., Schulman J. A., Smith P. A., Weight F. F., Walker R. J. Do cyclic nucleotides mediate slow postsynaptic potentials in sympathetic ganglia? [proceedings]. Br J Pharmacol. 1978 Mar;62(3):378P–379P. [PMC free article] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F., Smith P. A. Synaptic potentials in sympathetic ganglia: are they mediated by cyclic nucleotides? Science. 1978 Jun 2;200(4345):1079–1081. doi: 10.1126/science.206964. [DOI] [PubMed] [Google Scholar]
- Cehovic G., Posternak T., Charollais E. A study of the bioloical activity and resistance to phosphodiesterase of some derivatives and analogues of cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:521–540. [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The beta-adrenoceptor of the human lymphocyte and human lung parenchyma. Br J Pharmacol. 1977 Jan;59(1):17–23. doi: 10.1111/j.1476-5381.1977.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer H., Johnson D. G., Hanbauer I., Silberstein S. D., Kopin I. J. Accumulation of adenosine 3',5'-monophosphate induced by catecholamines in the rat superior cervical ganglion in vitro. Brain Res. 1973 Apr 13;53(1):97–104. doi: 10.1016/0006-8993(73)90769-5. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. Interactions between the catecholamines and ganglionic stimulating agents in sympathetic ganglia. J Pharmacol Exp Ther. 1966 Nov;154(2):200–215. [PubMed] [Google Scholar]
- Dun N. J., Kaibara K., Karczmar A. G. Dopamine and adenosine 3',5'-monophosphate responses of single mammalian sympathetic neurons. Science. 1977 Aug 19;197(4305):778–780. doi: 10.1126/science.196332. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. A comparison of the effect of theophylline and cyclic adenosine 3': 5'-monophosphate on the superior cervical ganglion of the rabbit by means of the sucrose-gap method. J Pharmacol Exp Ther. 1977 Jul;202(1):89–96. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Hayashi E., Mori M., Yamada S., Kumitomo M. Effects of purine compounds on cholinergic nerves. Specificity of adenosine and related compounds on acetylcholine release in electircally stimulated guinea pig ileum. Eur J Pharmacol. 1978 Apr 1;48(3):297–307. doi: 10.1016/0014-2999(78)90088-2. [DOI] [PubMed] [Google Scholar]
- Kalix P., McAfee D. A., Schorderet M., Greengard P. Pharmacological analysis of synaptically mediated increase in cyclic adenosine monophosphate in rabbit superior cervical ganglion. J Pharmacol Exp Ther. 1974 Mar;188(3):676–687. [PubMed] [Google Scholar]
- Kebabian J. W., Blood F. E., Steiner A. L., Greengard P. Neurotransmitters increase cyclic nucleotides in postganglionic neurons: immunocytochemical demonstration. Science. 1975 Oct 10;190(4210):157–159. doi: 10.1126/science.241121. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Hashiguchi T., Ushiyama N. Postsynaptic modulation of excitatory process in sympathetic ganglia by cyclic AMP. Nature. 1978 Jan 19;271(5642):268–270. doi: 10.1038/271268a0. [DOI] [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl T., Cramer H. Evidence against dopamine as the mediator of the rise of cyclic AMP in the superior cervical ganglion of the rat. Biochem Biophys Res Commun. 1975 Jul 22;65(2):731–739. doi: 10.1016/s0006-291x(75)80206-3. [DOI] [PubMed] [Google Scholar]
- Lindl T., Cramer H. Formation, accumulation and release of adenosine 3',5'-monophosphate induced by histamine in the superior cervical ganglion of the rat in vitro. Biochim Biophys Acta. 1974 Mar 20;343(1):182–191. doi: 10.1016/0304-4165(74)90250-5. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- Otten U., Mueller R. A., Oesch F., Thoenen H. Location of an isoproterenol-responsive cyclic AMP pool in adrenergic nerve cell bodies and its relationship to tyrosine 3-monooxygenase induction. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2217–2221. doi: 10.1073/pnas.71.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Kostopoulos G. K., Limacher J. J. Depression of corticospinal cells by various purines and pyrimidines. Can J Physiol Pharmacol. 1974 Dec;52(6):1226–1229. doi: 10.1139/y74-162. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Scholfield C. N. Depression of evoked potentials in brain slices by adenosine compounds. Br J Pharmacol. 1978 Jun;63(2):239–244. doi: 10.1111/j.1476-5381.1978.tb09752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard H., Wiggan G., Tsien W. H. Structure-activity relationships for inhibitors of phosphodiesterase from erythrocytes and other tissues. Adv Cyclic Nucleotide Res. 1972;1:103–112. [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]


