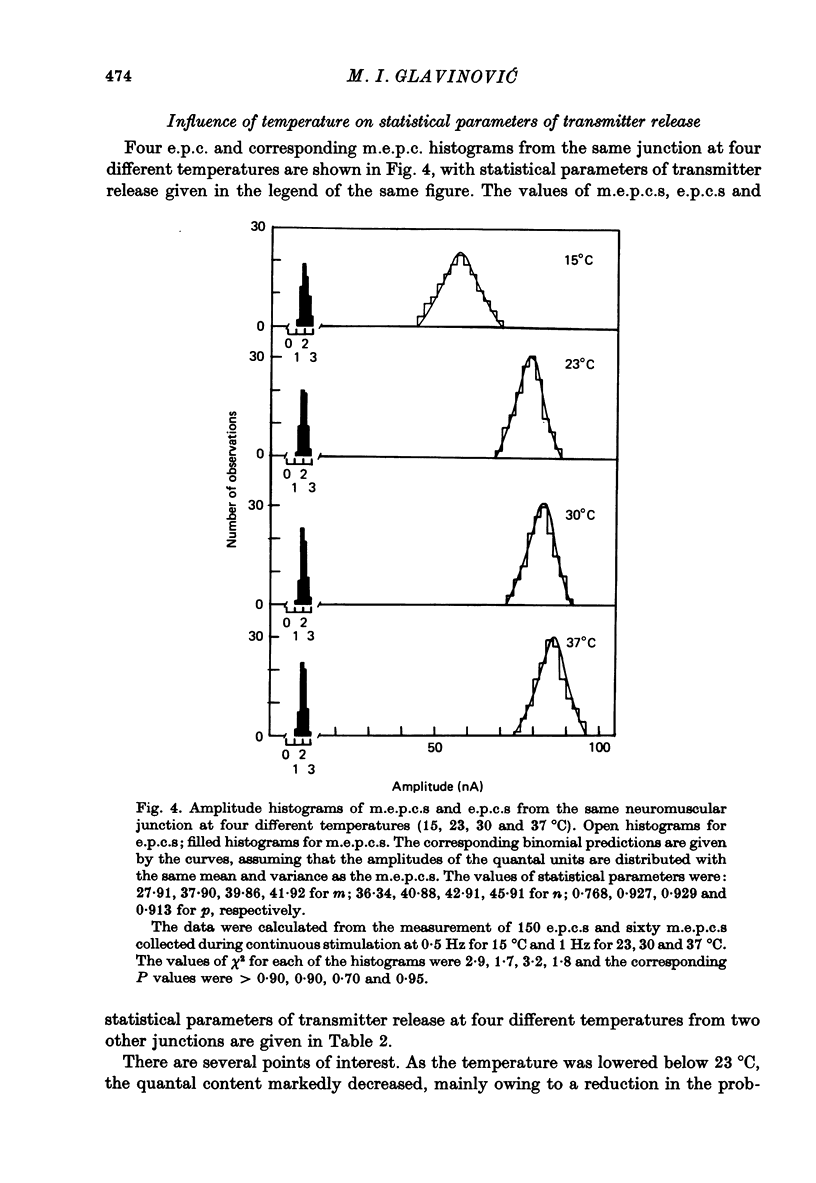
Abstract
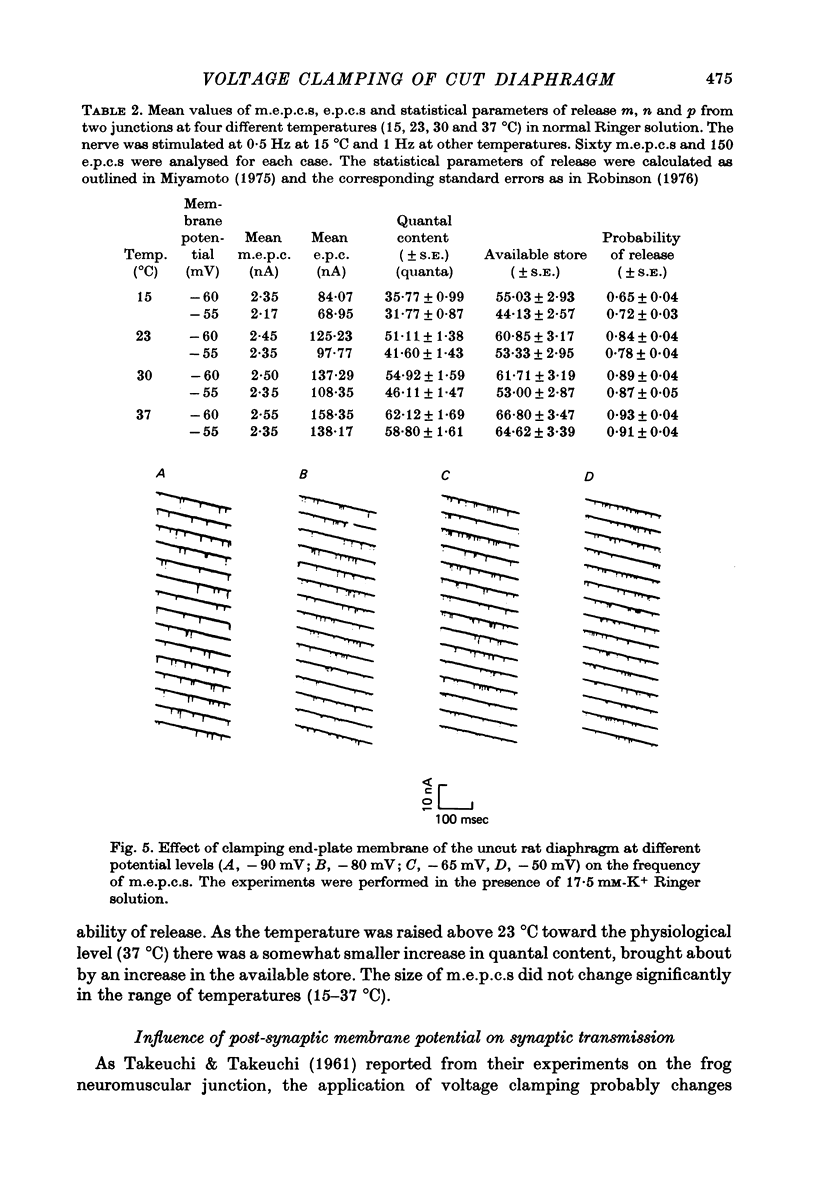
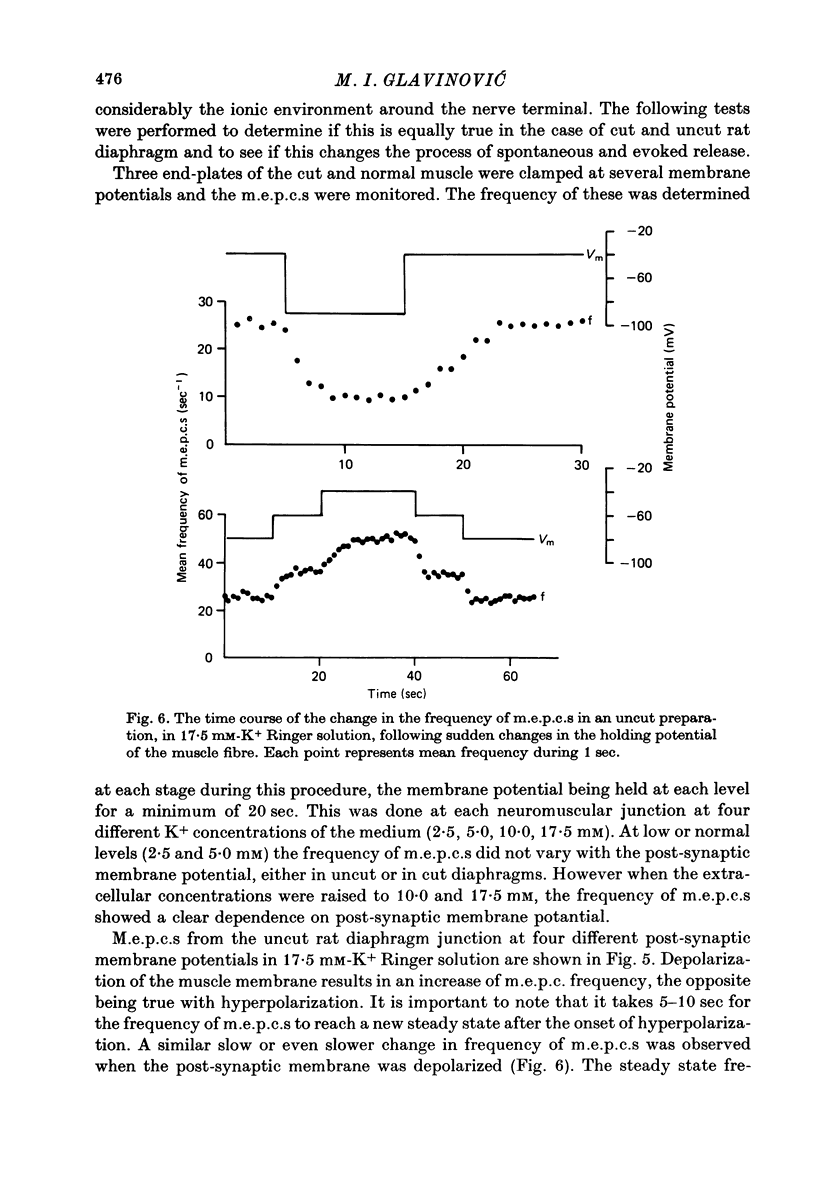
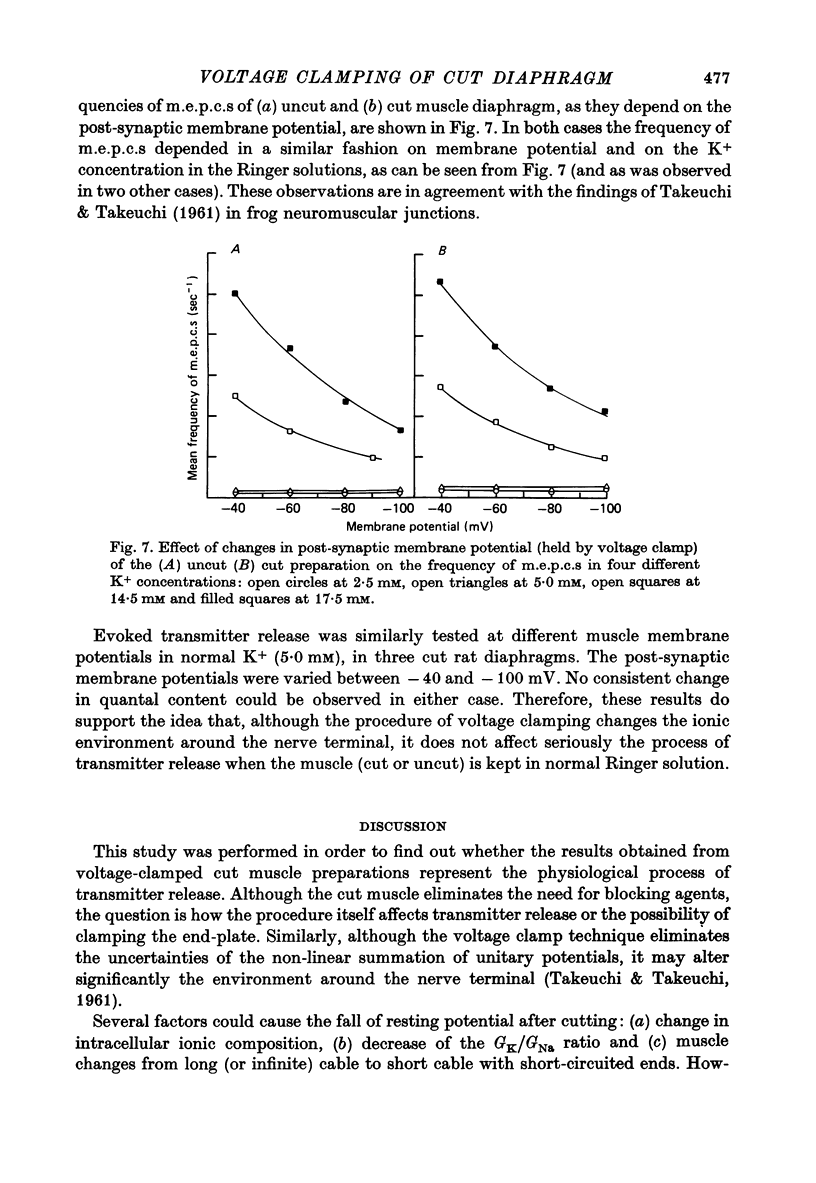
1. As a result of the cutting procedure there is a decrease in the membrane potential, input resistance, and space constant of the rat diaphragm, and there is no contraction when the phrenic nerve is stimulated. While the reversal potential of end-plate currents in cut preparations appears normal, the size of miniature end-plate currents (m.e.p.c.s.) is slightly decreased. 2. An increase in the external concentration of potassium from 2.5 to 10.0 mM results only in a minor change (less than 5%) in statistical parameters of transmitter release (m, n and p). The size of m.e.p.c. is also almost unchanged. 3. A decrease in temperature from 37 degrees to 15 degrees C resulted in a decrease of m, n and p; however, the values are less than 25% smaller at 25 degrees C than at 37 degrees C. The size of m.e.p.c.s is very insensitive to changes in temperature. 4. As previously reported for the frog neuromuscular junction, changes in muscle membrane potential of cut and uncut rat diaphragm due to voltage clamping affect the frequency of m.e.p.c.s only in medium with raised external K concentration. The dependence of frequency of m.e.p.c.s on muscle membrane potential is remarkably similar in cut and uncut preparations. Evoked release in cut preparation in normal medium is not affected by the change in muscle membrane potential.
Full text
PDF


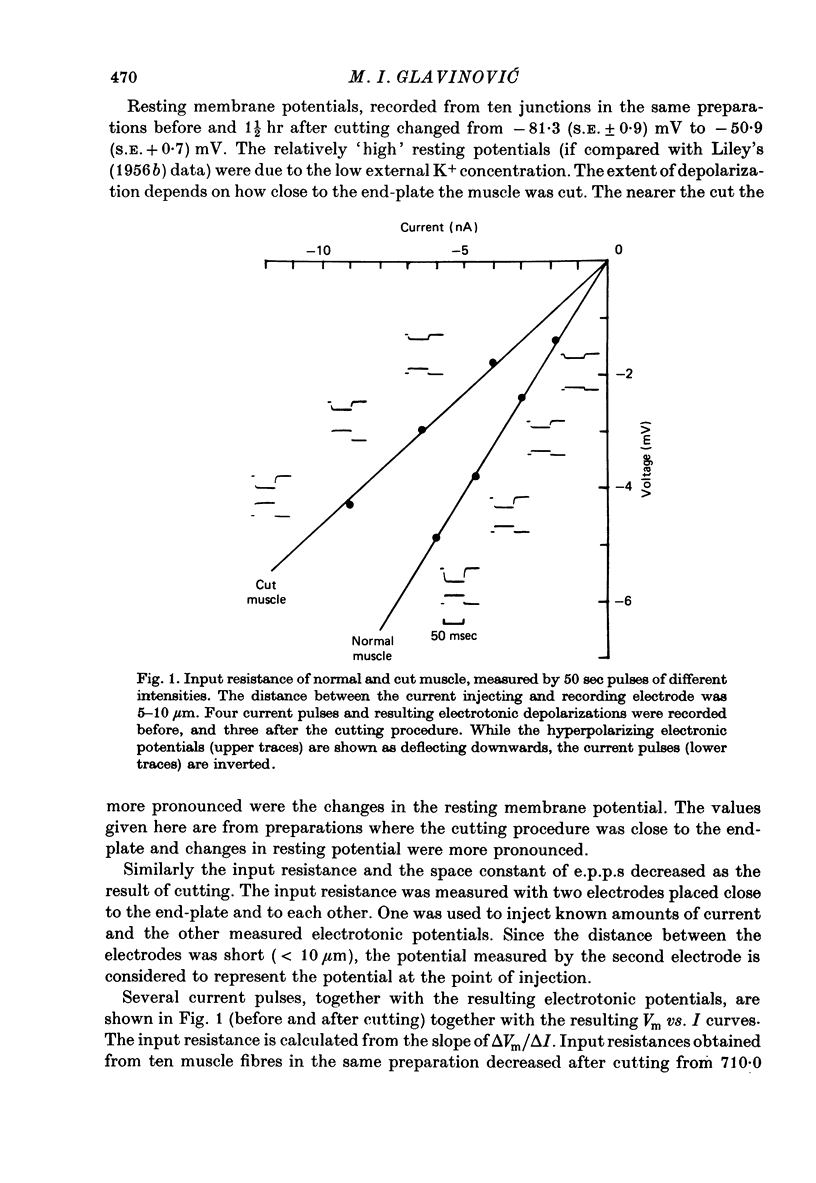
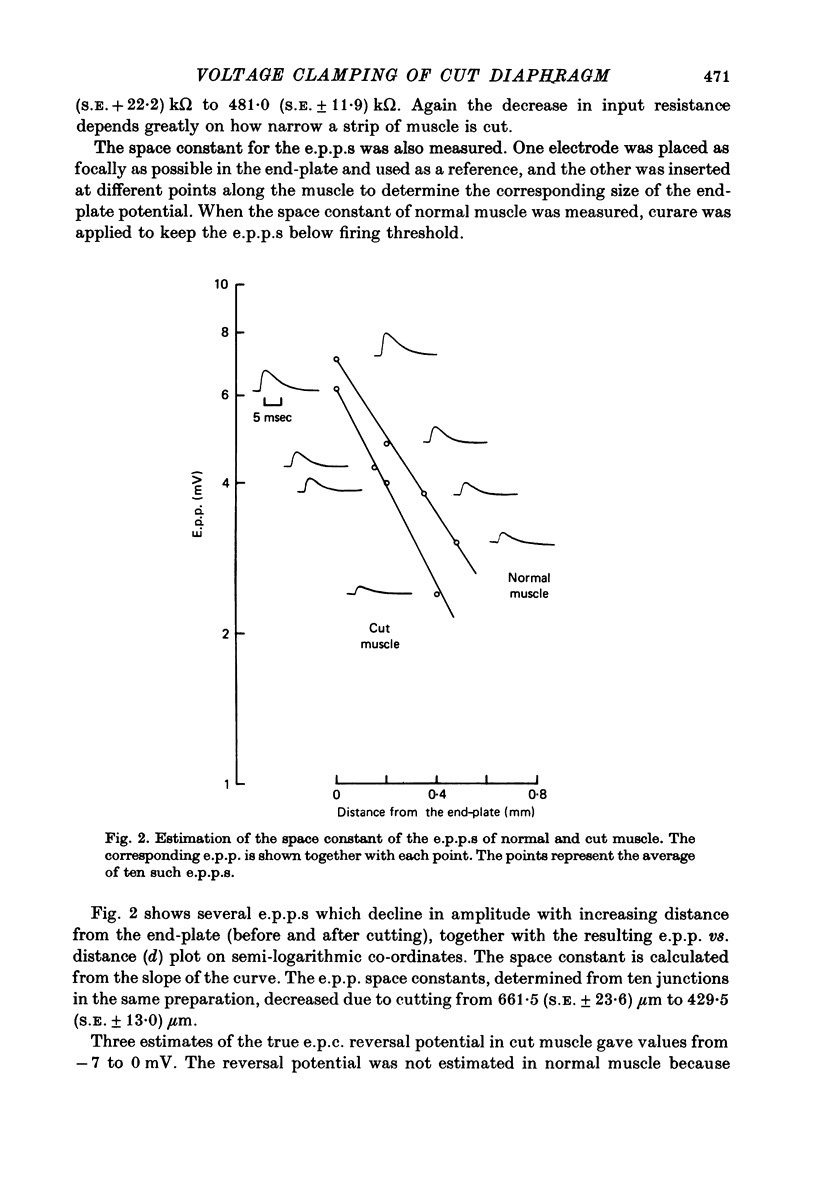
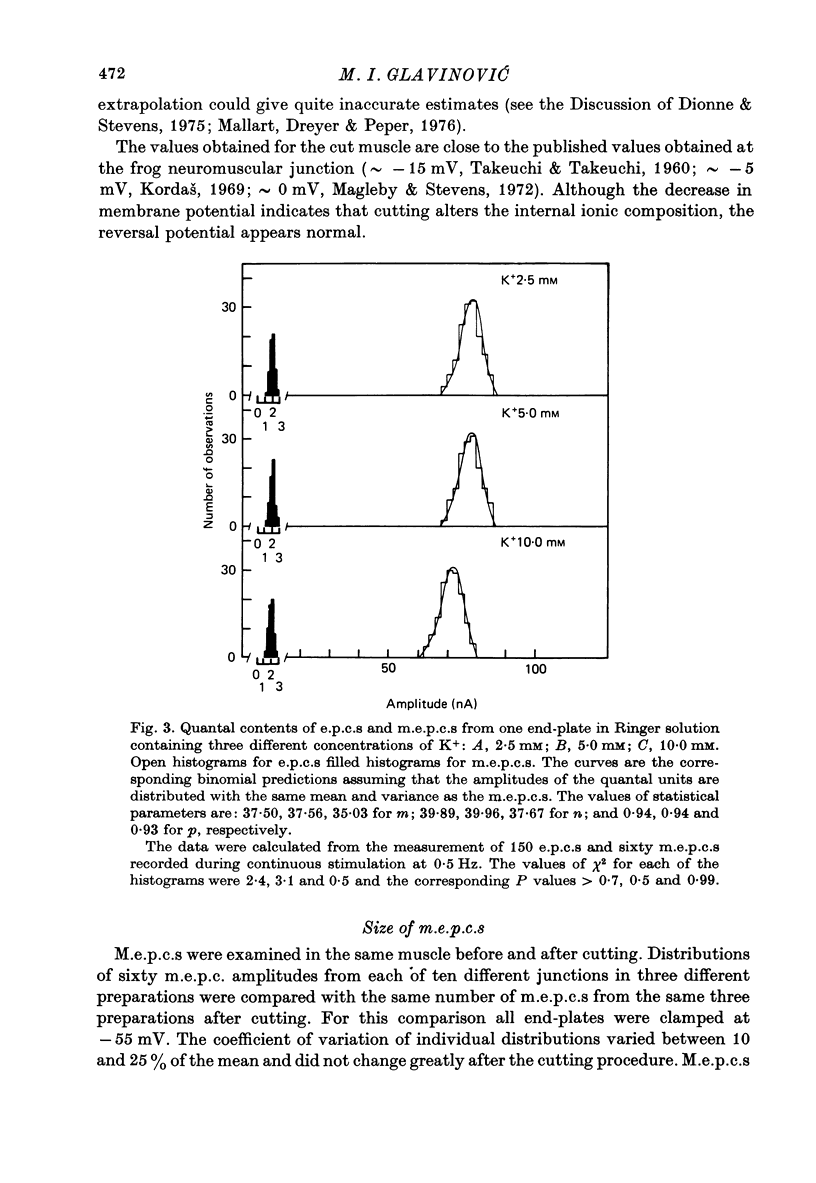
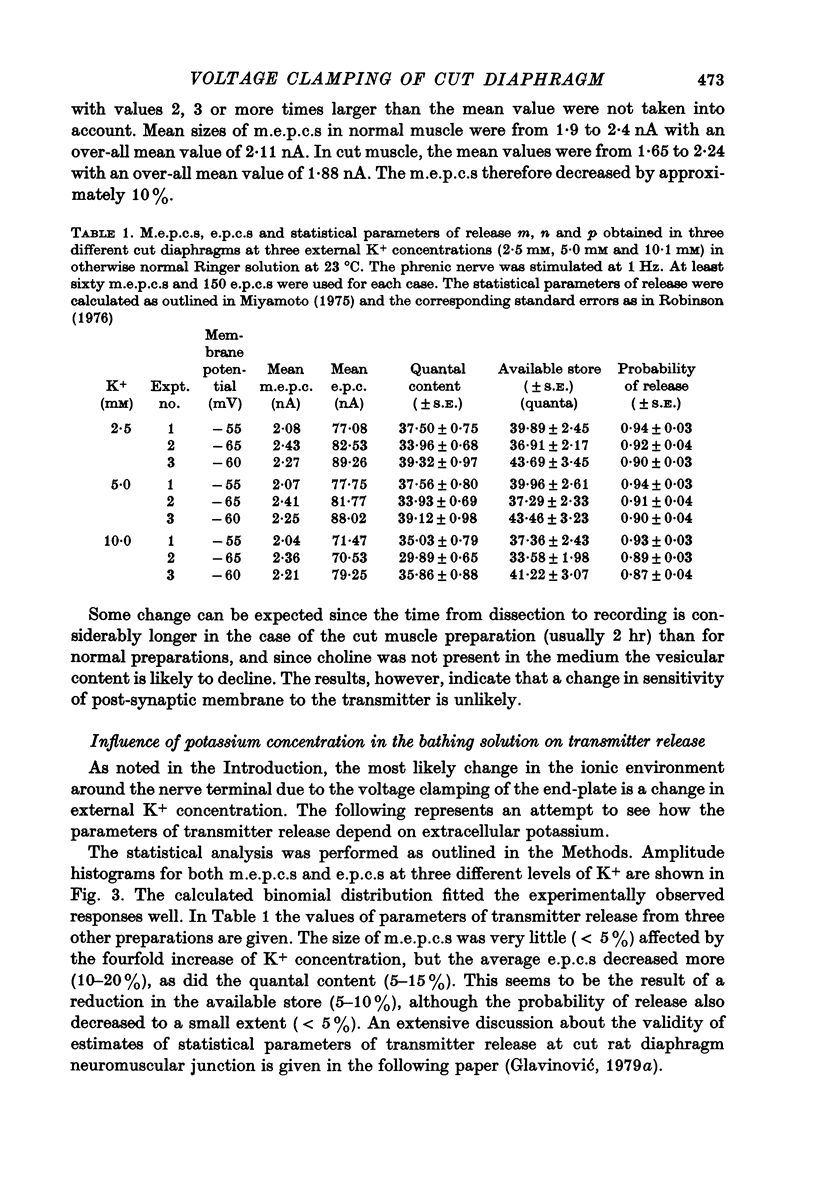



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- Branisteanu D. D., Miyamoto M. D., Volle R. L. Effects of physiologic alterations on binomial transmitter release at magnesium-depressed neuromuscular junctions. J Physiol. 1976 Jan;254(1):19–37. doi: 10.1113/jphysiol.1976.sp011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. T., Flaming D. G. Beveling of fine micropipette electrodes by a rapid precision method. Science. 1974 Aug;185(4152):693–695. doi: 10.1126/science.185.4152.693. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Erulkar S. D. Synaptic transmission and effects of temperature at the squid giant synapse. Nature. 1976 Jun 24;261(5562):720–722. doi: 10.1038/261720a0. [DOI] [PubMed] [Google Scholar]
- Zolovick A. J., Norman R. L., Fedde M. R. Membrane constants of muscle fibers of rat diaphragm. Am J Physiol. 1970 Sep;219(3):654–657. doi: 10.1152/ajplegacy.1970.219.3.654. [DOI] [PubMed] [Google Scholar]


