Abstract
1. The ionic conductances underlying some of the electrophysiological properties of multiply innervated or tonic fibres of rat extraocular muscles were examined in vitro with double-barrelled micro-electrodes.
2. Exposure of the muscle to a Cl-free saline did not change the effective resistance (Reff) of tonic fibres which was 5·14 ± 0·45 MΩ (n = 7) in control saline and 4·78 ± 0·45 MΩ (n = 12) in Cl-free saline (P > 0·1). In contrast, in singly innervated or twitch fibres Cl removal increased Reff from 1·77 ± 0·21 MΩ (n = 19) to 2·69 ± 0·12 MΩ (n = 22) (P < 0·001).
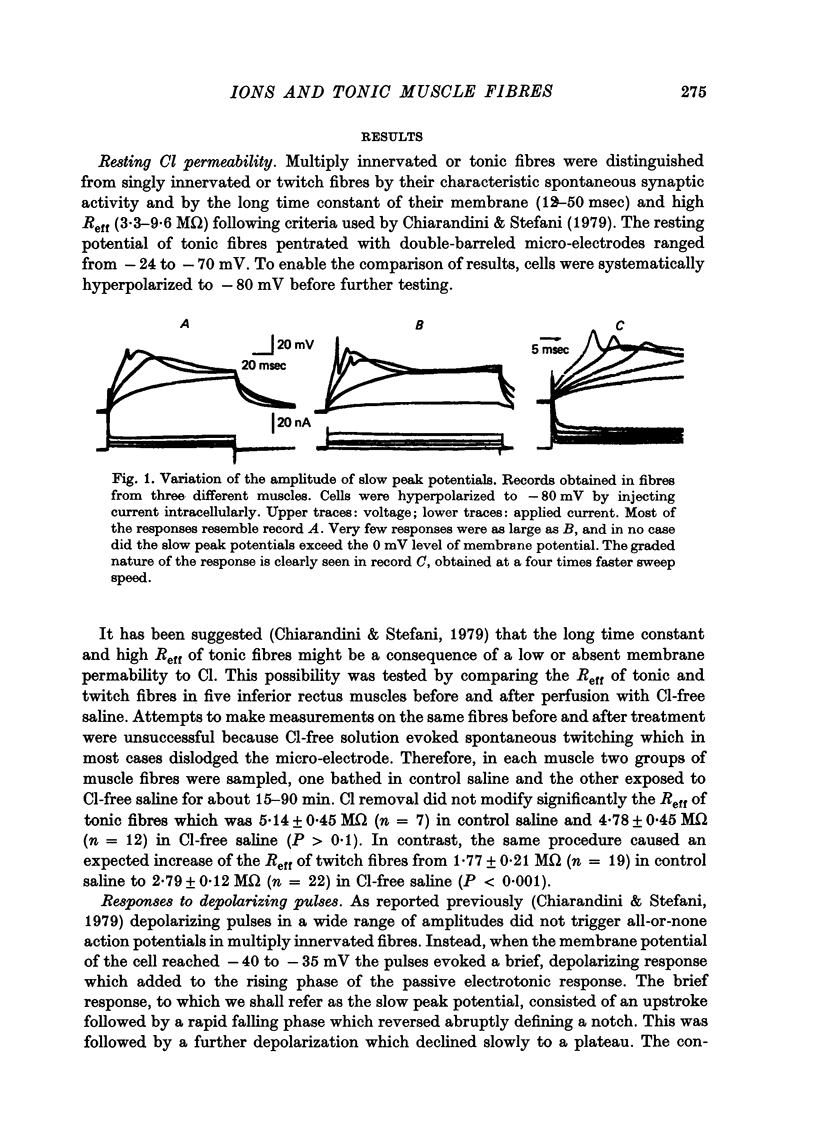
3. Tonic fibres with membrane potentials restored to - 80 mV by injecting current responded to intracellular depolarizing pulses with a brief, slow response (slow peak potential) which added to the rising phase of the electrotonic potential. The slow peak potential began at a membrane potential of - 40 to - 35 mV and was graded. Increasing depolarizations evoked faster and larger responses which did not over-shoot the zero level of membrane potential.
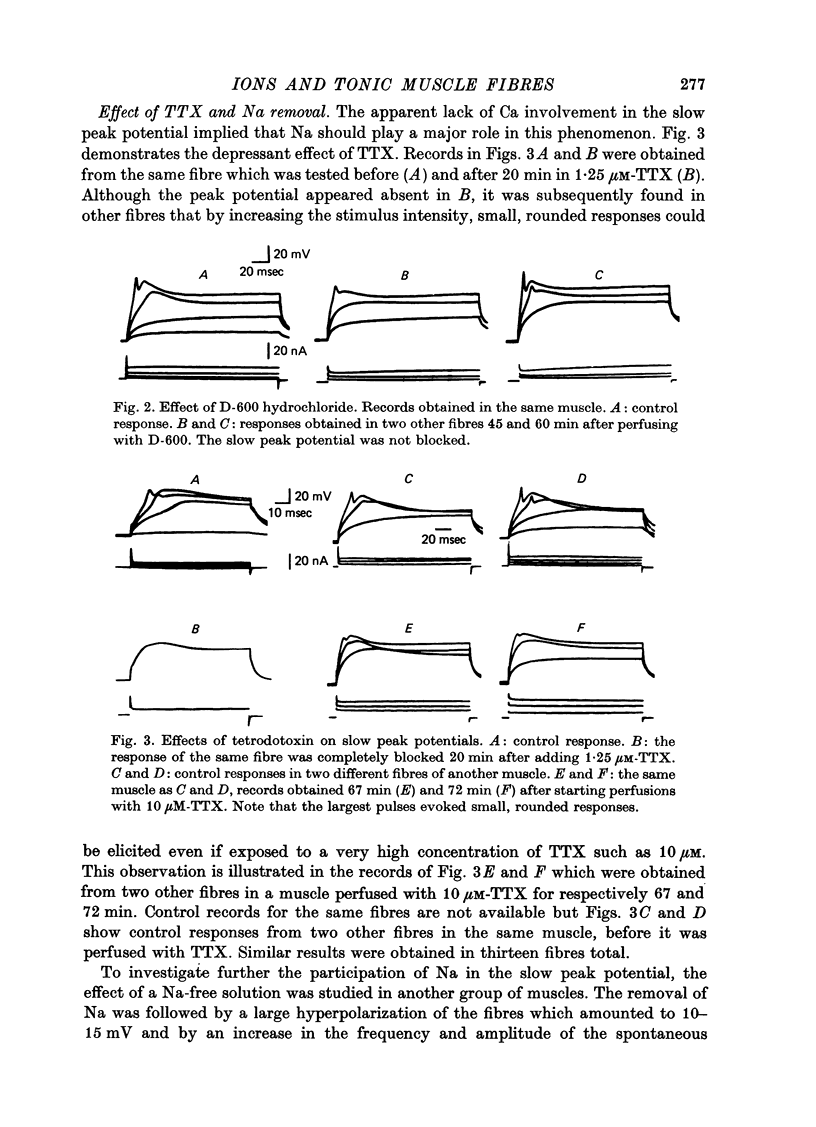
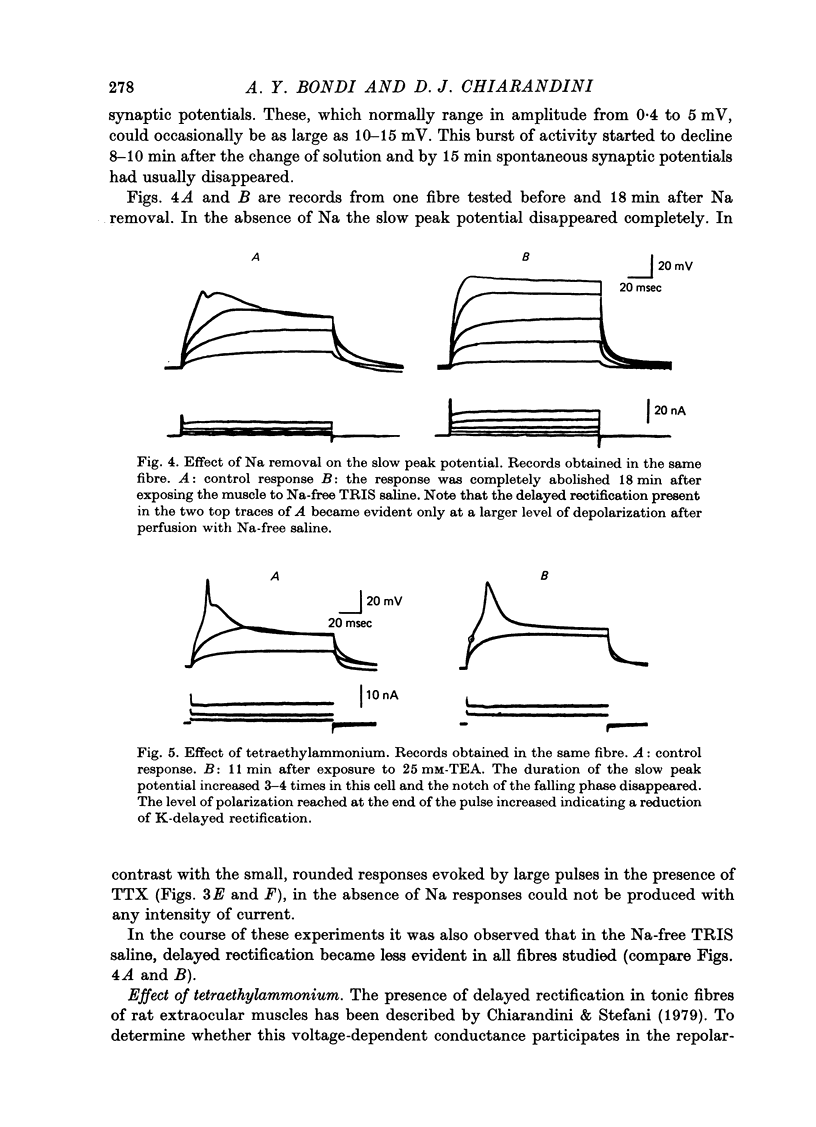
4. The slow peak potential was not blocked by 10 μM-D-600 hydrochloride but was markedly reduced by the absence of Na and by 10 μM-tetrodotoxin. The response was broadened about five times by 25 mM-tetraethylammonium.
5. Raising bath temperature from 21-25 °C to 37 °C reversibly depressed and shortened the slow peak potential but did not transform it into an action potential.
6. It is concluded that the characteristic high Reff of tonic fibres results from a lack of a membrane conductance to Cl and that the slow peak potential involves the transient activation of Na and K channels which are pharmacologically similar to the respective channels of twitch fibres.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-y-Rita P., Ito F. In vivo studies on fast and slow muscle fibers in cat extraocular muscles. J Gen Physiol. 1966 Jul;49(6):1177–1198. doi: 10.1085/jgp.0491177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack N. H., Bell C. C., Rence B. G. Tension and rate of tension development during isometric responses of extraocular muscle. J Neurophysiol. 1971 Nov;34(6):1072–1079. doi: 10.1152/jn.1971.34.6.1072. [DOI] [PubMed] [Google Scholar]
- Chiarandini D. J., Davidowitz J. Structure and function of extraocular muscle fibers. Curr Top Eye Res. 1979;1:91–142. [PubMed] [Google Scholar]
- Chiarandini D. J., Stefani E. Electrophysiological identification of two types of fibres in rat extraocular muscles. J Physiol. 1979 May;290(2):453–465. doi: 10.1113/jphysiol.1979.sp012783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörrscheidt-Käfer M. The action of D600 on frog skeletal muscle: facilitation of excitation-contraction coupling. Pflugers Arch. 1977 Jul 19;369(3):259–267. doi: 10.1007/BF00582193. [DOI] [PubMed] [Google Scholar]
- Fernand V. S., Hess A. The occurrence, structure and innervation of slow and twitch muscle fibres in the tensor tympani and stapedius of the cat. J Physiol. 1969 Feb;200(2):547–554. doi: 10.1113/jphysiol.1969.sp008707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K. Cholinesterase activity in sheep oesophageal muscle. J Anat. 1973 Dec;116(Pt 3):357–373. [PMC free article] [PubMed] [Google Scholar]
- HESS A., PILAR G. SLOW FIBRES IN THE EXTRAOCULAR MUSCLES OF THE CAT. J Physiol. 1963 Dec;169:780–798. doi: 10.1113/jphysiol.1963.sp007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATYUSHKIN D. P. MOTOR SYSTEMS IN THE OCULOMOTOR APPARATUS OF HIGHER ANIMALS. Fed Proc Transl Suppl. 1964 Sep-Oct;23:1103–1106. [PubMed] [Google Scholar]
- Mayr R. Structure and distribution of fibre types in the external eye muscles of the rat. Tissue Cell. 1971;3(3):433–462. doi: 10.1016/s0040-8166(71)80045-9. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilar G. Further study of the electrical and machanical responses of slow fibers in cat extraocular muscles. J Gen Physiol. 1967 Oct;50(9):2289–2300. doi: 10.1085/jgp.50.9.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]


