Abstract
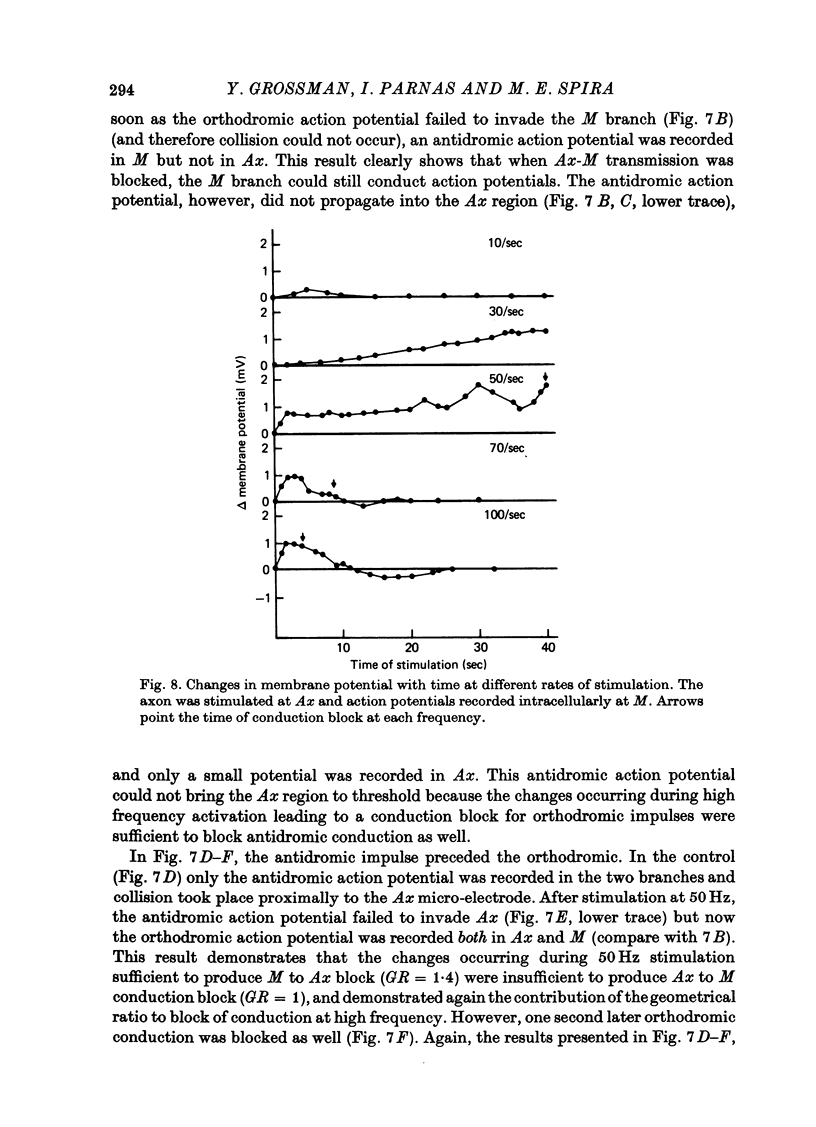
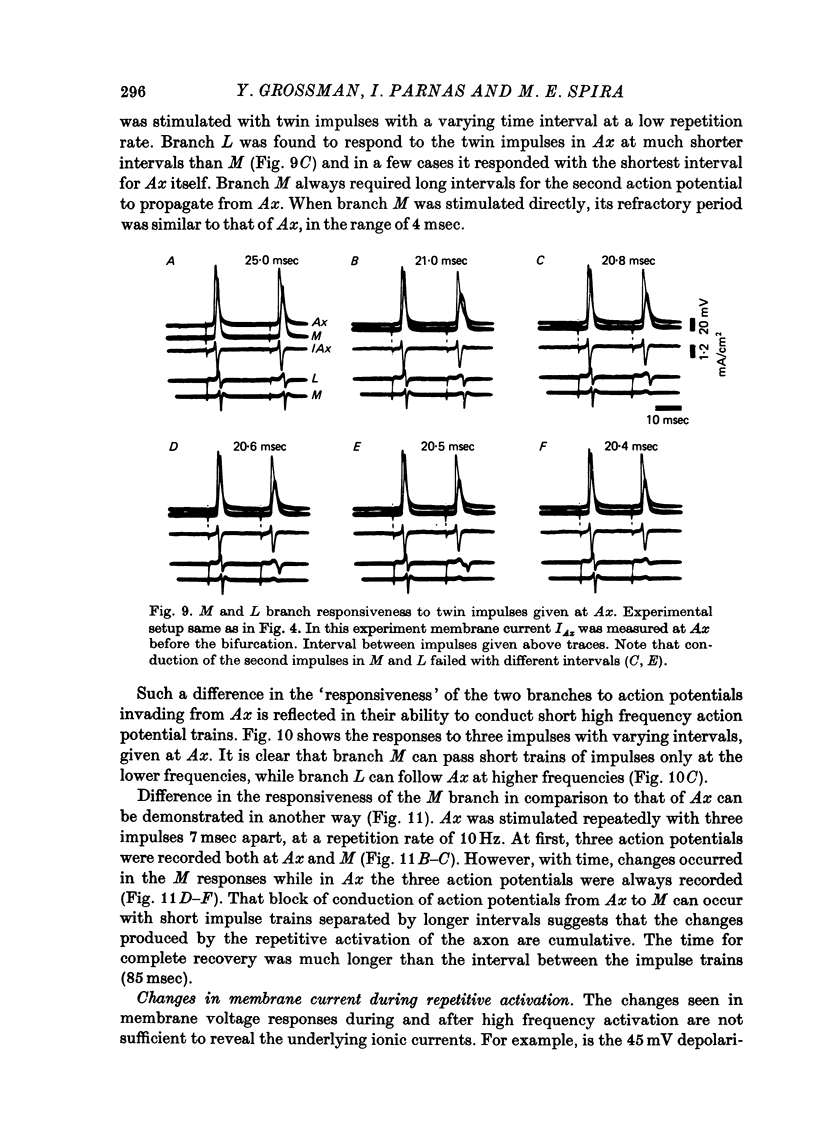
1. Propagation of action potentials at high frequency was studied in a branching axon of the lobster by means of simultaneous intracellular recording both before and after the branch point. 2. Although the branching axon studied has a geometrical ratio close to one (perfect impedance matching) conduction across the branch point failed at stimulation frequencies above 30 Hz. 3. The block of conduction after high frequency stimulation occurred at the branch point per se. The parent axon and daughter branches continued to conduct action potentials. 4. Conduction block after high frequency stimulation appeared first in the thicker daughter branch and only later in the thin branch. 5. With high frequency stimulation there was a 10-15% reduction in amplitude of the action potential in the parent axon, a corresponding decrease in the rate of rise of the action potential, a 25-30% decrease in conduction velocity, marked increase in threshold and prolongation of the refractory period. In addition the membrane was depolarized by 1-3 mV. 6. Measurements of the membrane current using the patch clamp technique showed a large decrease in the phase of inward current associated with the action potential, before the branching point. 7. The small membrane depolarization seen after high frequency stimulation is not the sole cause of the conduction block. Imposed prolonged membrane depolarization (8 mV for 120 sec) was insufficient to produce conduction block. 8. In vivo chronic extracellular recordings from the main nerve bundle (which contains the parent axon) and the large daughter branch revealed that: (a) the duration and frequency of trains of action potentials along the axons exceeded those used in the isolated nerve experiments and (b) conduction failure in the large daughter branch could be induced in the whole animal by electrical stimulation of the main branch as in the isolated preparation. 9. Possible mechanisms underlying block of conduction after high frequency stimulation in a branching axon are discussed.
Full text
PDF


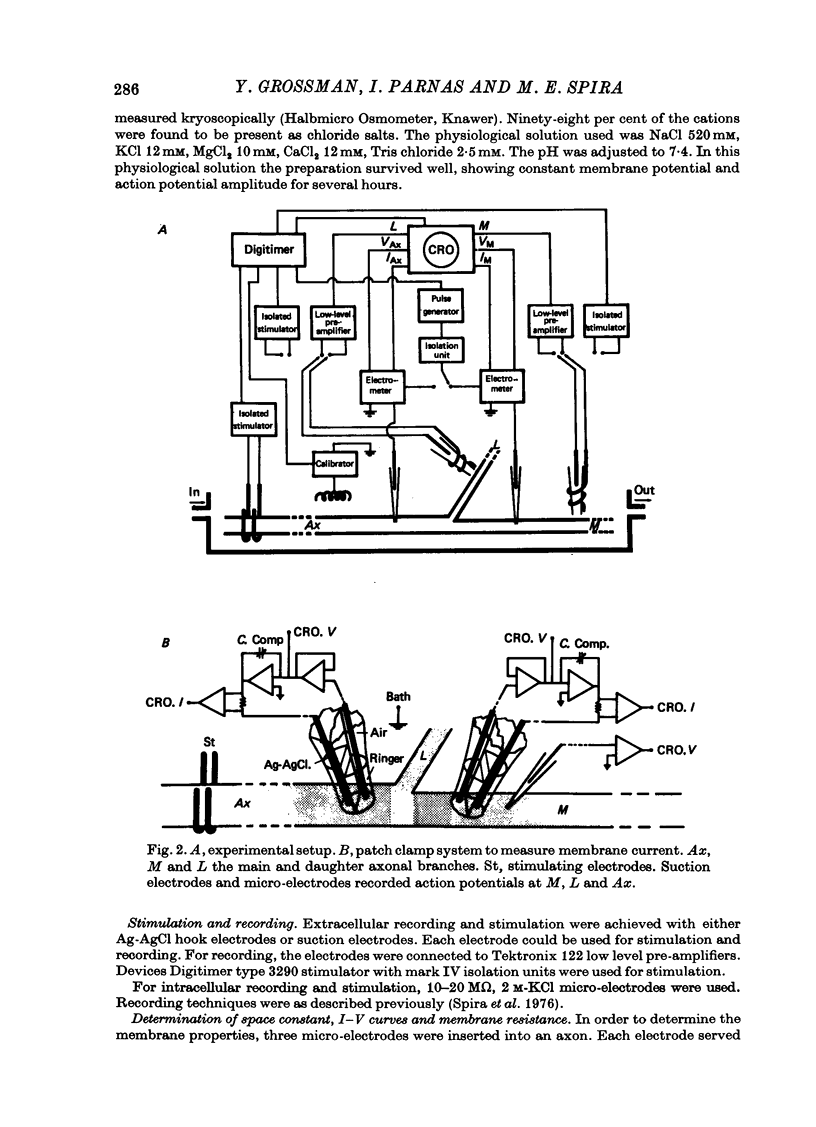

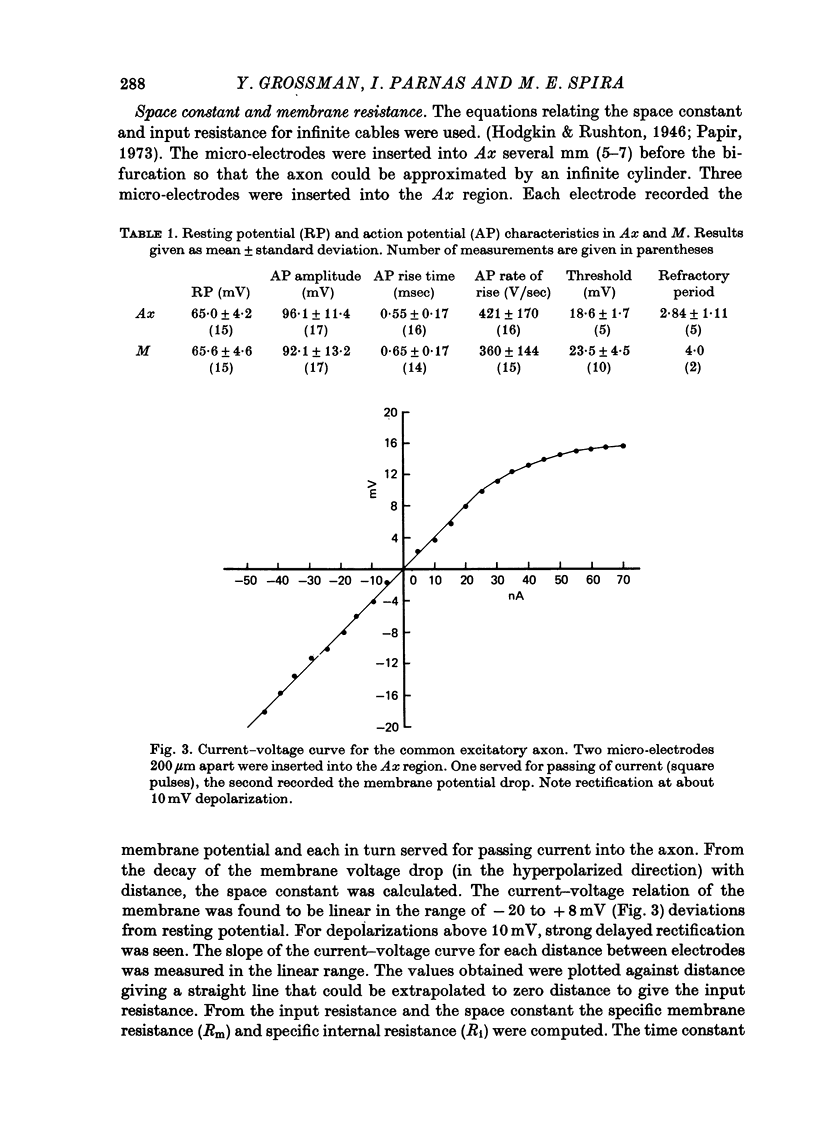
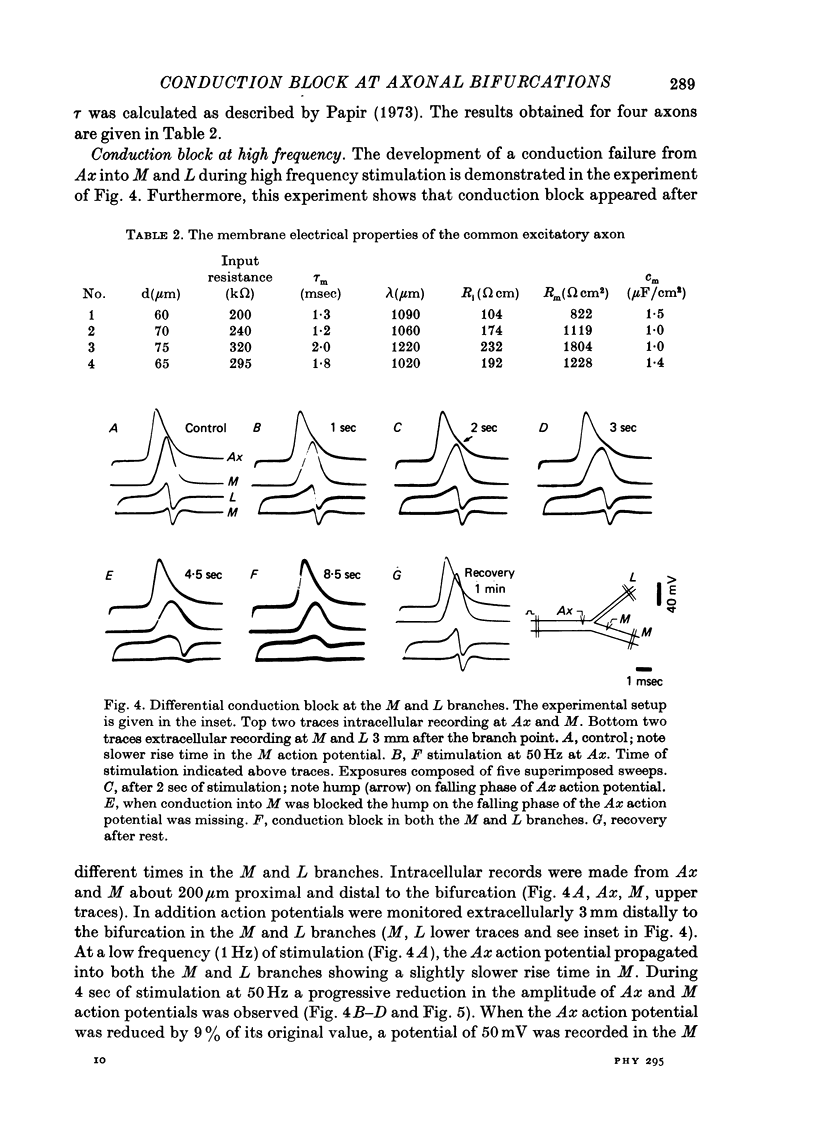
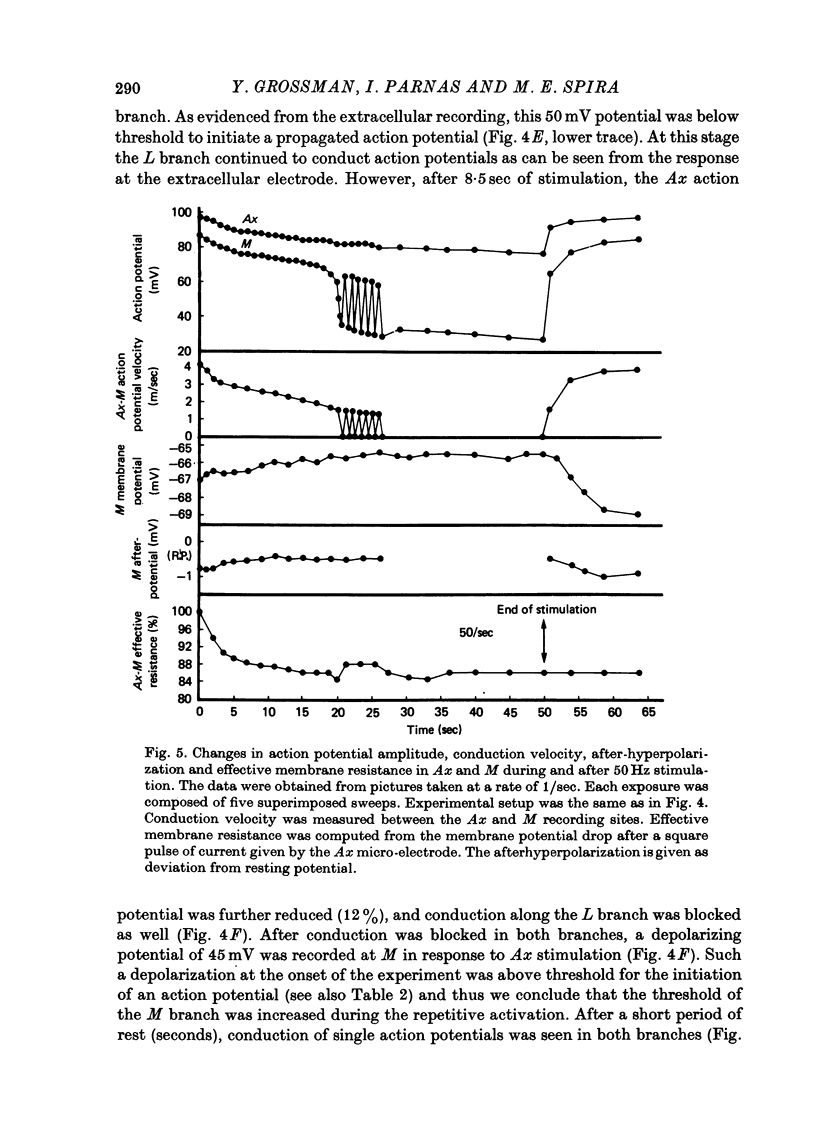













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Raymond S. A., Lettvin J. Y. Multiple meaning in single visual units. Brain Behav Evol. 1970;3(1):72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. S., Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J. 1974 Oct;14(10):731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Parnas I., Spira M. E. Mechanisms involved in differential conduction of potentials at high frequency in a branching axon. J Physiol. 1979 Oct;295:307–322. doi: 10.1113/jphysiol.1979.sp012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Axon conduction block: differential channeling of nerve impulses in the crayfish. Brain Res. 1975 Apr 4;87(1):85–88. doi: 10.1016/0006-8993(75)90784-2. [DOI] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY D., MELLON D., Jr SYNAPTIC ACTIVATION AND RECEPTIVE FIELDS IN CRAYFISH INTERNEURONS. Comp Biochem Physiol. 1964 Dec;13:275–300. doi: 10.1016/0010-406x(64)90025-8. [DOI] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Voltage clamp on Helix pomatia neuronal membrane; current measurement over a limited area of the soma surface. Pflugers Arch. 1969;311(3):272–277. doi: 10.1007/BF00590532. [DOI] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev. 1966 Jan;46(1):1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]
- Papir D. The effect of glycerol treatment on crab muscle fibres. J Physiol. 1973 Apr;230(2):313–330. doi: 10.1113/jphysiol.1973.sp010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Atwood H. L. Phasic and tonic neuromuscular systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol. 1966 Aug;18(4):701–723. doi: 10.1016/0010-406x(66)90206-4. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Parnas I., Hochstein S., Parnas H. Theoretical analysis of parameters leading to frequency modulation along an inhomogeneous axon. J Neurophysiol. 1976 Jul;39(4):909–923. doi: 10.1152/jn.1976.39.4.909. [DOI] [PubMed] [Google Scholar]
- Parnas I., Segev I. A mathematical model for conduction of action potentials along bifurcating axons. J Physiol. 1979 Oct;295:323–343. doi: 10.1113/jphysiol.1979.sp012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Ramón F., Joyner R. W., Moore J. W. Propagation of action potentials in inhomogeneous axon regions. Fed Proc. 1975 Apr;34(5):1357–1363. [PubMed] [Google Scholar]
- Spira M. E., Yarom Y., Parnas I. Modulation of spike frequency by regions of special axonal geometry and by synaptic inputs. J Neurophysiol. 1976 Jul;39(4):882–899. doi: 10.1152/jn.1976.39.4.882. [DOI] [PubMed] [Google Scholar]
- TAUC L., HUGHES G. M. Modes of initiation and propagation of spikes in the branching axons of molluscan central neurons. J Gen Physiol. 1963 Jan;46:533–549. doi: 10.1085/jgp.46.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973 May;230(3):509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE A., TAKEDA K. The spread of excitation among neurons in the heart ganglion of the stomatopod, Squillia oratoria. J Gen Physiol. 1963 Mar;46:773–801. doi: 10.1085/jgp.46.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S. G. Integrative properties and design principles of axons. Int Rev Neurobiol. 1975;18:1–40. doi: 10.1016/s0074-7742(08)60032-x. [DOI] [PubMed] [Google Scholar]
- Waxman S. G. Regional differentiation of the axon: a review with special reference to the concept of the multiplex neuron. Brain Res. 1972 Dec 12;47(2):269–288. doi: 10.1016/0006-8993(72)90639-7. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976 Dec;263(3):513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]



