Abstract
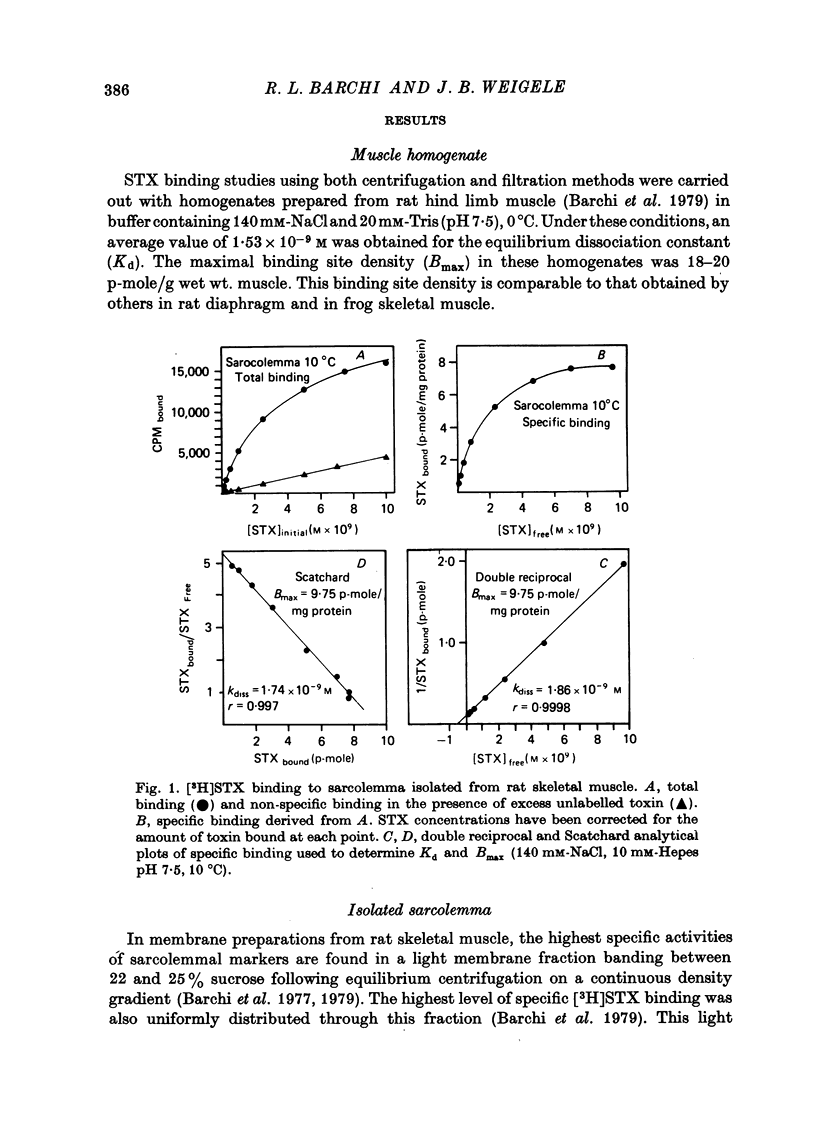
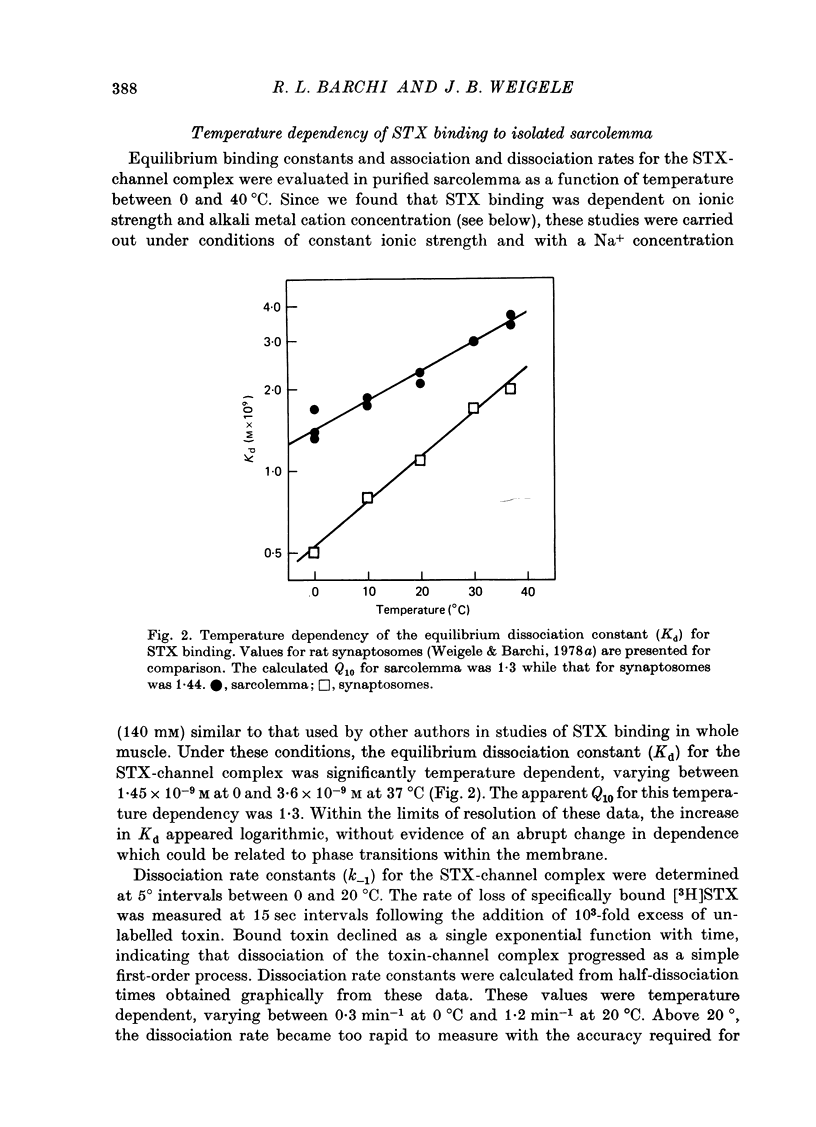
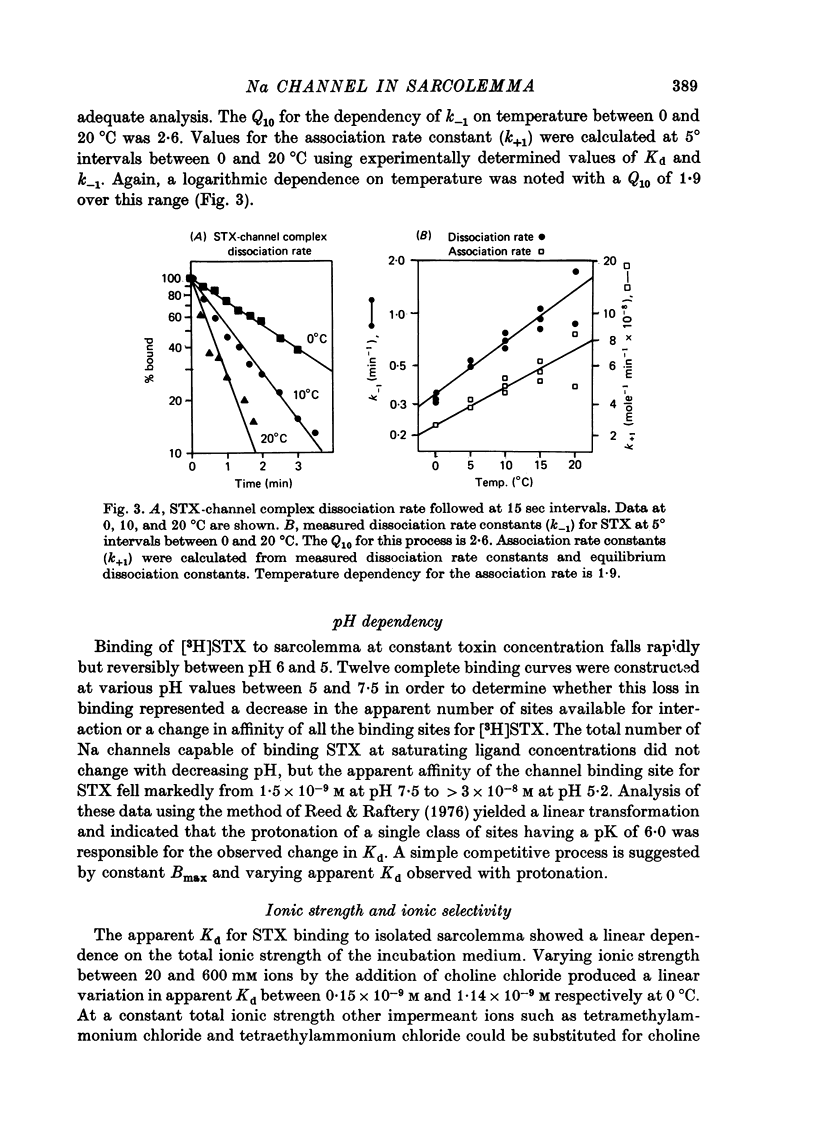
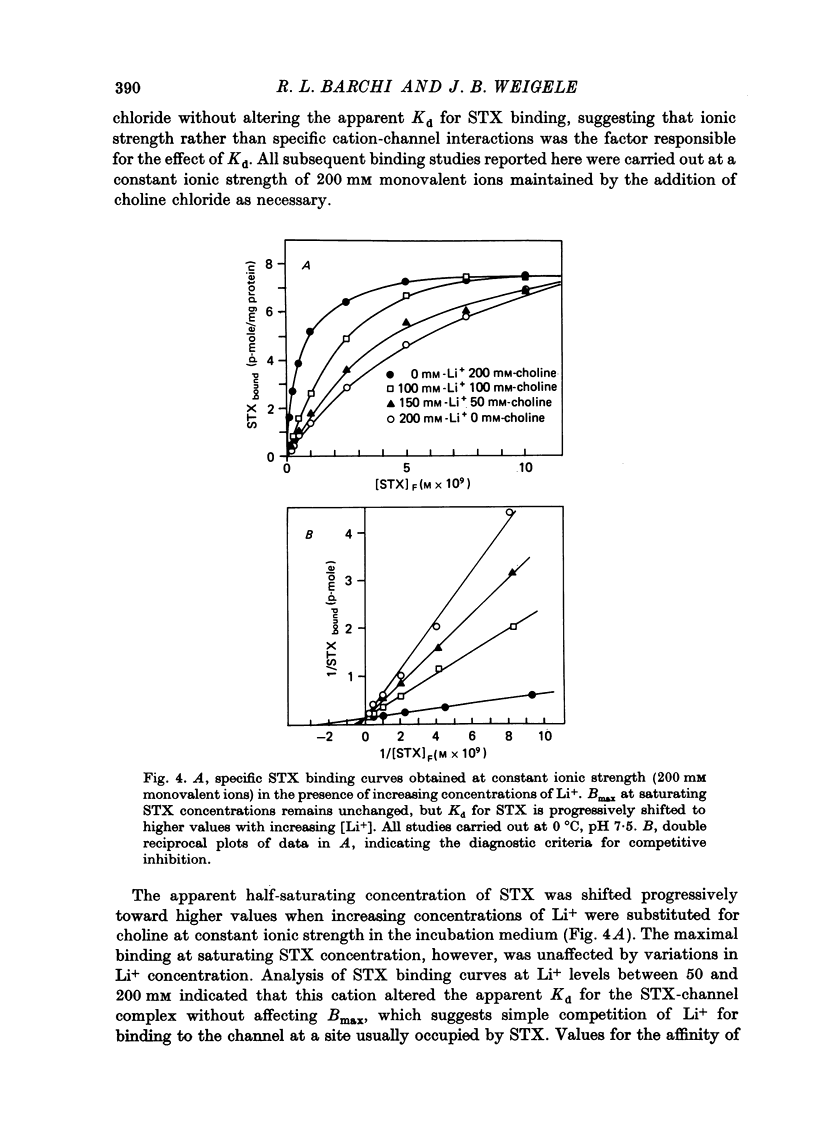
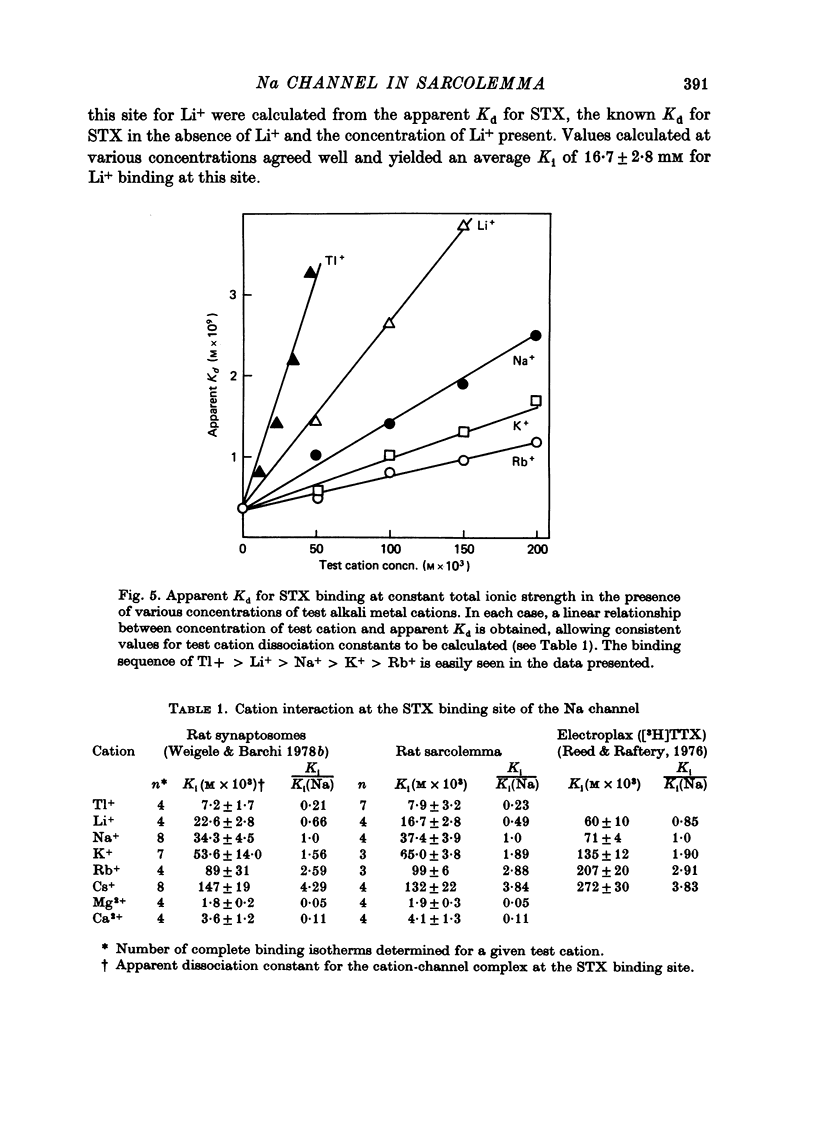
1. The characteristics of saxitoxin (STX) binding to the mammalian Na channel have been studied in purified sarcolemma isolated from rat skeletal muscle. 2. STX binds specifically to isolated sarcolemma with a Kd of 1.43 x 10(-9) M and Bmax of 7-8 p-mole STX bound/mg membrane protein at 0 degrees C in the presence of 140 mM-NaCl. In rat muscle homogenate under the same conditions the corresponding values are Kd = 1.53 x 10(-9) M and Bmax = 0.15-0.20 p-mole/mg protein (18-20 p-mole/g wet wt.). Membrane purification produced a fortyfold increase in STX binding site concentration per milligram protein. Calculated binding site density in isolated sarcolemma was about 30 sites/micron 2 of membrane surface. 3. Denervation (10-14 days) results in a 43% reduction in the density of high-affinity STX binding sites in purified sarcolemma, but the Kd for this class of sites is not changed. 4. In sarcolemma, the apparent Kd for STX binding is dependent on temperature pH and ionic strength. The Q10 for Kd between 0 and 40 degrees C is 1.3. Protonation of a group having a pK of 6.0 markedly raises Kd without affecting Bmax. Apparent Kd increases eightfold when ionic strength is raised from 20 to 600 mM. 5. Dissociation and association rate constants for STX binding are temperature dependent with Q10 of 2.6 and 1.9 respectively between 0 and 20 degrees C. 6. STX binding is competitively inhibited by monovalent and divalent cations under conditions of constant total ionic strength. An affinity sequence of Tl+ greater than Li+ greater than Na+ greater than K+ greater than Rb+ greater than Cs+ is seen for the monovalent cation-binding site. 7. The STX binding site is relatively stable to heat and to enzymic degradation. A specific modifier of carboxyl residues inactivates subsequent STX binding. This process can be prevented by the presence of STX during the reaction. 8. Characteristics of the STX binding site in isolated sarcolemma are compared to those reported for other isolated excitable membranes and for studies of whole muscle and muscle homogenate. Sarcolemma provides a potential source of enriched Na channels for further purification efforts in a mammalian system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Levinson S. R. Tetrodotoxin binding to normal depolarized frog muscle and the conductance of a single sodium channel. J Physiol. 1975 May;247(2):483–509. doi: 10.1113/jphysiol.1975.sp010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Balerna M., Fosset M., Chicheportiche R., Romey G., Lazdunski M. Constitution and properties of axonal membranes of crustacean nerves. Biochemistry. 1975 Dec 16;14(25):5500–5511. doi: 10.1021/bi00696a019. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Bonilla E., Wong M. Isolation and characterization of muscle membranes using surface-specific labels. Proc Natl Acad Sci U S A. 1977 Jan;74(1):34–38. doi: 10.1073/pnas.74.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B., Chalikian D. M., Murphy L. E. Muscle surface membranes: preparative methods affect apparent chemical properties and neurotoxin binding. Biochim Biophys Acta. 1979 Jan 5;550(1):59–76. doi: 10.1016/0005-2736(79)90115-9. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J Biol Chem. 1977 Dec 10;252(23):8669–8676. [PubMed] [Google Scholar]
- Chacko G. K., Barnola F. V., Villegas R., Goldman D. E. The binding of tetrodotoxin to axonal membrane fraction isolated from garfish olfactory nerve. Biochim Biophys Acta. 1974 Dec 10;373(2):308–312. doi: 10.1016/0005-2736(74)90154-0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Rang H. P., Ritchie J. M. The binding of tetrodotoxin and alpha-bungarotoxin to normal and denervated mammalian muscle. J Physiol. 1974 Jul;240(1):199–226. doi: 10.1113/jphysiol.1974.sp010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festoff B. W., Engel W. K. In vitro analysis of the general properties and junctional receptor characteristics of skeletal muscle membranes. Isolation, purification, and partial characterization of sarcolemmal fragments. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2435–2439. doi: 10.1073/pnas.71.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemann D. R. Binding of radioactive tetrodotoxin to nerve membrane preparations. Biochim Biophys Acta. 1972 May 9;266(2):548–556. doi: 10.1016/0005-2736(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Venosa R. A., Shrager P., Horowicz P. Density and distribution of tetrodotoxin receptors in normal and detubulated frog sartorius muscle. J Gen Physiol. 1976 Apr;67(4):399–416. doi: 10.1085/jgp.67.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Reed J. K., Raftery M. A. Properties of the tetrodotoxin binding component in plasma membranes isolated from Electrophorus electricus. Biochemistry. 1976 Mar 9;15(5):944–953. doi: 10.1021/bi00650a002. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. Binding of tetrodotoxin and saxitoxin to sodium channels. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):319–336. doi: 10.1098/rstb.1975.0012. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Characterization of exchange-labeled saxitoxin and the origin of linear uptake by excitable tissue. Mol Pharmacol. 1977 Nov;13(6):1136–1146. [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of labelled saxitoxin to normal and denervated muscle [proceedings]. J Physiol. 1976 Dec;263(1):129P–130P. [PubMed] [Google Scholar]
- Shrager P., Profera C. Inhibition of the receptor for tetrodotoxin in nerve membranes by reagents modifying carboxyl groups. Biochim Biophys Acta. 1973 Aug 9;318(1):141–146. doi: 10.1016/0005-2736(73)90343-x. [DOI] [PubMed] [Google Scholar]
- Strong P. N., Smith J. T., Keana J. F. A convenient bioassay for detecting nanomolar concentrations of tetrodotoxin. Toxicon. 1973 Aug;11(5):433–438. doi: 10.1016/0041-0101(73)90119-0. [DOI] [PubMed] [Google Scholar]
- Villegas R., Barnola F. V., Camejo G. Action of proteases and phospholipases on tetrodotoxin binding to axolemma preparations isolated from lobster nerve fibres. Biochim Biophys Acta. 1973 Aug 9;318(1):61–68. doi: 10.1016/0005-2736(73)90336-2. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Analysis of saxitoxin binding in isolated rat synaptosomes using a rapid filtration assay. FEBS Lett. 1978 Jul 15;91(2):310–314. doi: 10.1016/0014-5793(78)81199-5. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Saxitoxin binding to the mammalian sodium channel. Competition by monovalent and divalent cations. FEBS Lett. 1978 Nov 1;95(1):49–53. doi: 10.1016/0014-5793(78)80049-0. [DOI] [PubMed] [Google Scholar]


