Abstract
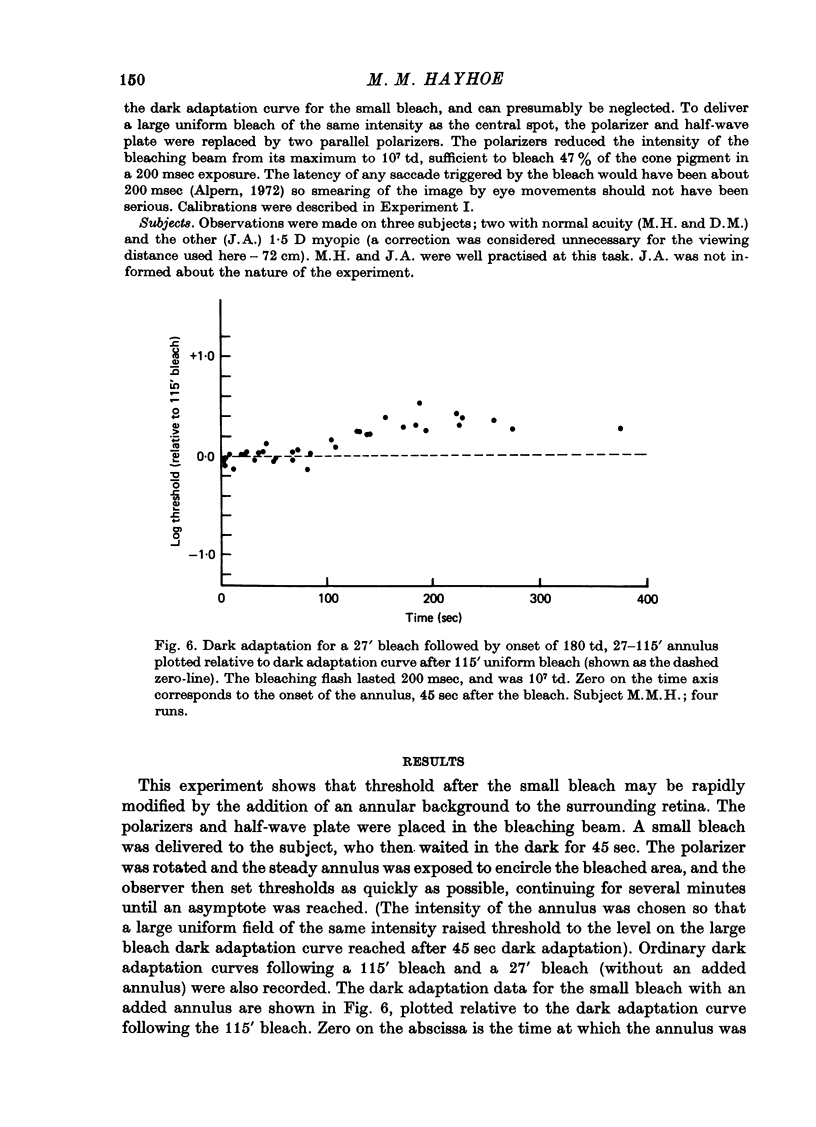
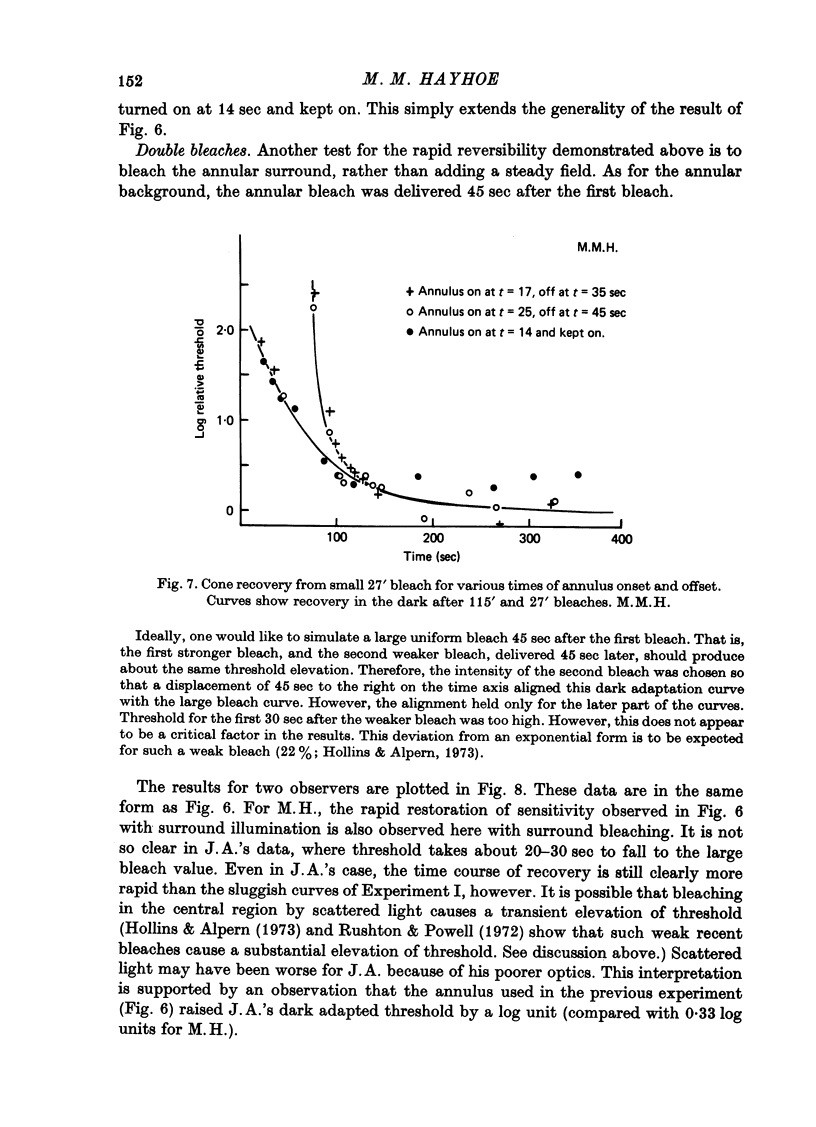
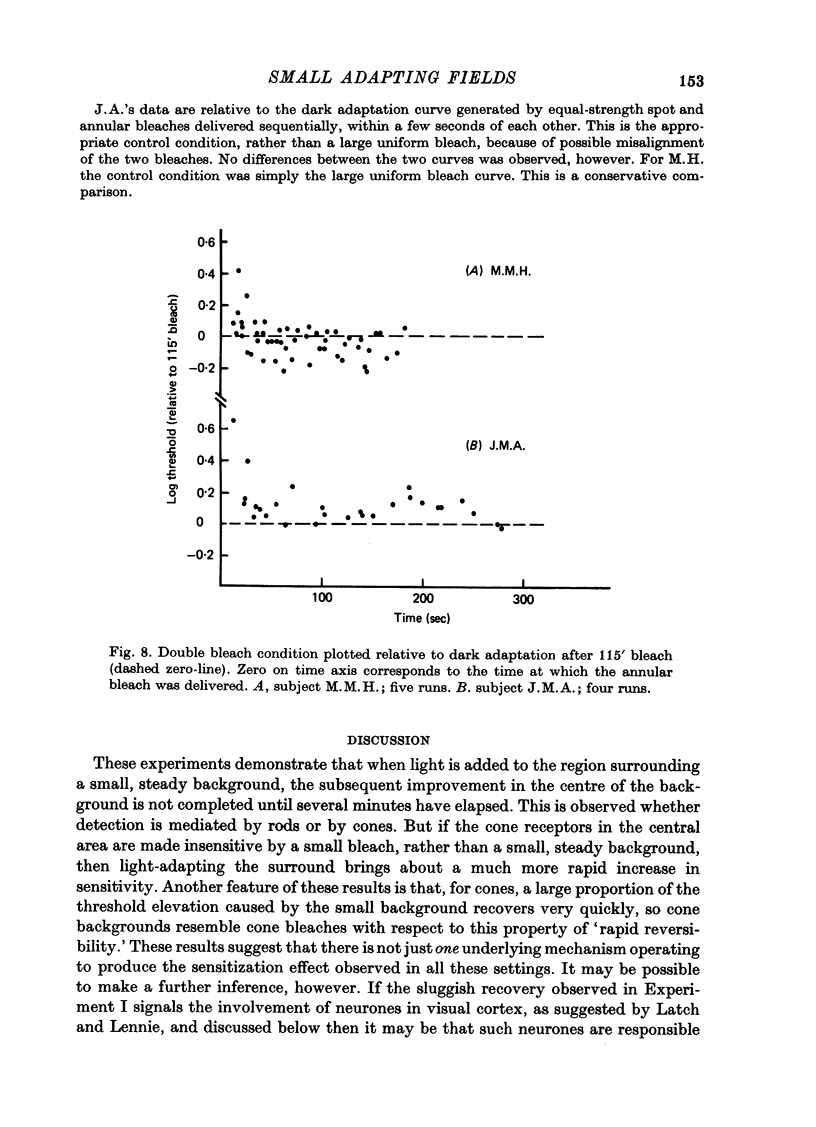
1. Sensitivity to a small test probe in the centre of a small, steady background is less than when the background is large (sensitization). When an equiluminous steady annulus is added to the region surrounding a small background, rod threshold takes several minutes to stabilize at its new, lower level. The after-effects of the small background follow a time course characteristic of cortical adaptation. 2. The sensitivity loss and time course of recovery after intense bleaching lights in the cone system depend markedly on the size of the retinal region bleached, although no such effect is observed in the rod system. If a steady annular surround is added to the region surrounding the bleached patch, threshold falls rapidly to the value it would have after a large-area bleach of the same intensity. 3. The interaction between bleaches and steady surrounds suggests that bleaches produce long-lasting signals in the cone receptors. 4. The different temporal properties of sensitization on rod backgrounds and sensitization after cone bleaches suggest that different mechanisms underlie the two phenomena. 5. In cone vision, if light is added to the area surrounding a small, steady background, the subsequent readjustment takes minutes to complete, as it does in rod vision. But in addition, for cones, a large proportion of the sensitivity loss caused by the small background can be rapidly restored, as it is with cone bleaches. 6. The above results, together with the known absence of sensitization in rod dark adaptation, are consistent with the hypothesis that sensitization occurs at least partly at the retinal level in the cone system, but not (or only weakly) in the rod system, and that there is an additional, probably cortical elevation, common to rod and cone systems, for small backgrounds, but not for small, brief bleaches.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M. EVIDENCE FOR A PHYSIOLOGICAL EXPLANATION OF THE WATERFALL PHENOMENON AND FIGURAL AFTER-EFFECTS. Nature. 1963 Dec 28;200:1345–1347. doi: 10.1038/2001345a0. [DOI] [PubMed] [Google Scholar]
- Baker H. D., Bargoot F. G. Effect of stimulus presentation rate upon visual threshold. Vision Res. 1977;17(3):379–383. doi: 10.1016/0042-6989(77)90027-x. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Sakitt B. Doubts about scotopic interactions in stabilized vision. Vision Res. 1973 Feb;13(2):523–524. doi: 10.1016/0042-6989(73)90136-3. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick D. W., MacLeod D. I. Rod threshold: influence of neighboring cones. Vision Res. 1978;18(12):1611–1616. doi: 10.1016/0042-6989(78)90252-3. [DOI] [PubMed] [Google Scholar]
- Bodinger D. M. The decay of grating adaptation. Vision Res. 1978;18(1):89–91. doi: 10.1016/0042-6989(78)90081-0. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Sensitization and centre-surround antagonism in Necturus retina. J Physiol. 1974 Feb;236(3):593–610. doi: 10.1113/jphysiol.1974.sp010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch J. M. Quantitative layer-by-layer perimetry. Invest Ophthalmol Vis Sci. 1978 Mar;17(3):208–257. [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkes T. E., Temme L. A. Rod-cone interaction in human scotopic vision--II. Cones influence rod increment thresholds. Vision Res. 1977;17(6):673–679. doi: 10.1016/s0042-6989(77)80001-1. [DOI] [PubMed] [Google Scholar]
- HAMMER E. R. Temporal factors in figural aftereffects. Am J Psychol. 1949 Jul;62(3):337–354. [PubMed] [Google Scholar]
- Hayhoe M. M. Lateral interactions in human cone dark adaptation. J Physiol. 1979 Nov;296:125–140. doi: 10.1113/jphysiol.1979.sp012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M., Alpern M. Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol. 1973 Oct;62(4):430–447. doi: 10.1085/jgp.62.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Johnson C. A., Enoch J. M. Human psychophysical analysis of receptive field-like properties--II. Dichoptic properties of the Westheimer function. Vision Res. 1976;16(12):1455–1462. doi: 10.1016/0042-6989(76)90165-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latch M., Lennie P. Rod-cone interaction in light adaptation. J Physiol. 1977 Aug;269(3):517–534. doi: 10.1113/jphysiol.1977.sp011912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P., MacLeod D. I. Background configuration and rod threshold. J Physiol. 1973 Aug;233(1):143–156. doi: 10.1113/jphysiol.1973.sp010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. I. Visual sensitivity. Annu Rev Psychol. 1978;29:613–645. doi: 10.1146/annurev.ps.29.020178.003145. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973 Dec 7;182(4116):1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Markoff J. I., Sturr J. F. Spatial and luminance determinants of the increment threshold under monoptic and dichoptic viewing. J Opt Soc Am. 1971 Nov;61(11):1530–1537. doi: 10.1364/josa.61.001530. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton W. A. Bleached rhodopson and visual adaptation. J Physiol. 1965 Dec;181(3):645–655. doi: 10.1113/jphysiol.1965.sp007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton W. A., Powell D. S. The rhodopsin content and the visual threshold of human rods. Vision Res. 1972 Jun;12(6):1073–1081. doi: 10.1016/0042-6989(72)90098-3. [DOI] [PubMed] [Google Scholar]
- Singer W., Zihl J., Pöppel E. Subcortical control of visual thresholds in humans: evidence for modality specific and retinotopically organized mechanisms of selective attention. Exp Brain Res. 1977 Aug 31;29(2):173–190. doi: 10.1007/BF00237040. [DOI] [PubMed] [Google Scholar]
- Sturr J. F., Teller D. Y. Sensitization by annular surrounds: dichoptic properties. Vision Res. 1973 May;13(5):909–918. doi: 10.1016/0042-6989(73)90071-0. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Gestrin P. J. Sensitization by annular surrounds: sensitization and dark adaptation. Vision Res. 1969 Dec;9(12):1481–1489. doi: 10.1016/0042-6989(69)90064-9. [DOI] [PubMed] [Google Scholar]
- Teller D. Y., Matter C., Phillips W. D., Alexander K. Sensitization by annular surrounds: sensitization and masking. Vision Res. 1971 Dec;11(12):1445–1458. doi: 10.1016/0042-6989(71)90065-4. [DOI] [PubMed] [Google Scholar]
- Teller D. Y. Sensitization by annular surrounds: temporal (masking) properties. Vision Res. 1971 Nov;11(11):1325–1335. doi: 10.1016/0042-6989(71)90014-9. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U., Jones R. M. Spatial sensitization as a function of delay. Vision Res. 1977;17(10):1191–1199. doi: 10.1016/0042-6989(77)90153-5. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U., Vassilev A. Foveal spatial sensitization with stabilized vision. Vision Res. 1974 Jan;14(1):101–105. doi: 10.1016/0042-6989(74)90122-9. [DOI] [PubMed] [Google Scholar]
- Vautin R. G., Berkley M. A. Responses of single cells in cat visual cortex to prolonged stimulus movement: neural correlates of visual aftereffects. J Neurophysiol. 1977 Sep;40(5):1051–1065. doi: 10.1152/jn.1977.40.5.1051. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler B. S. The electroretinogram of the isolated rat retina. Vision Res. 1972 Jun;12(6):1183–1198. doi: 10.1016/0042-6989(72)90106-x. [DOI] [PubMed] [Google Scholar]


