Abstract
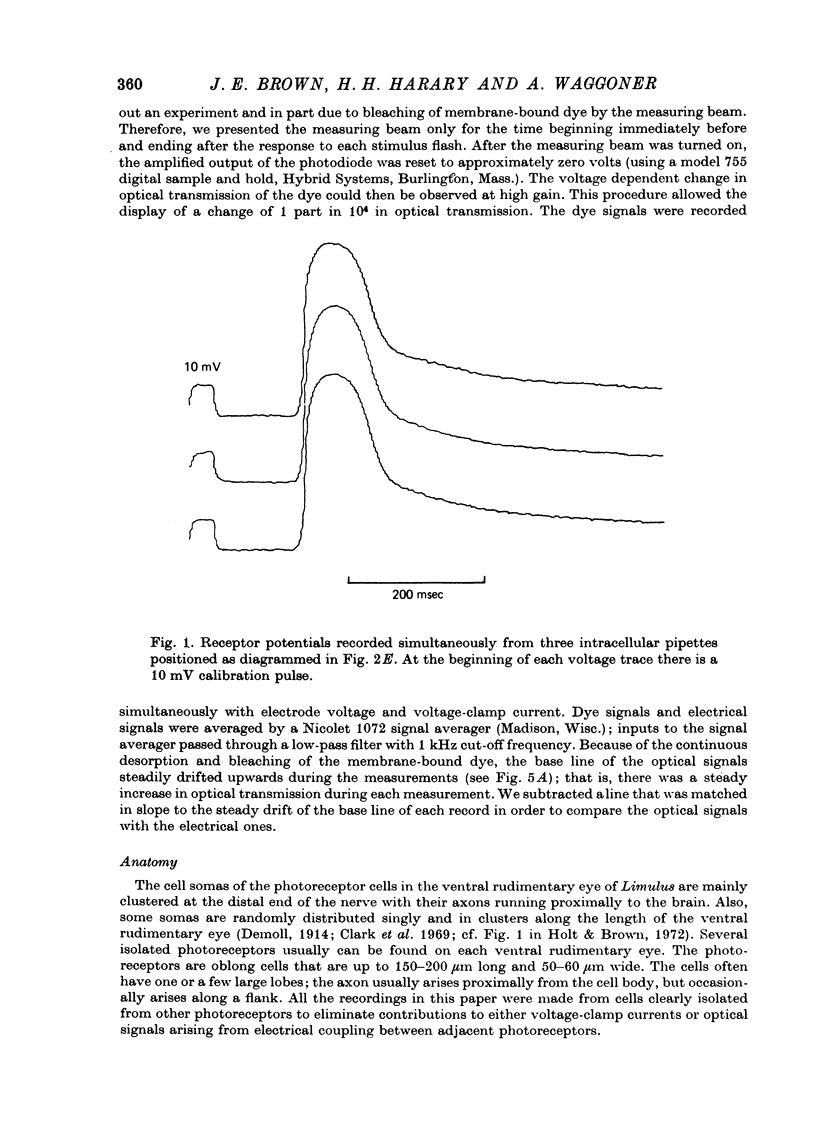
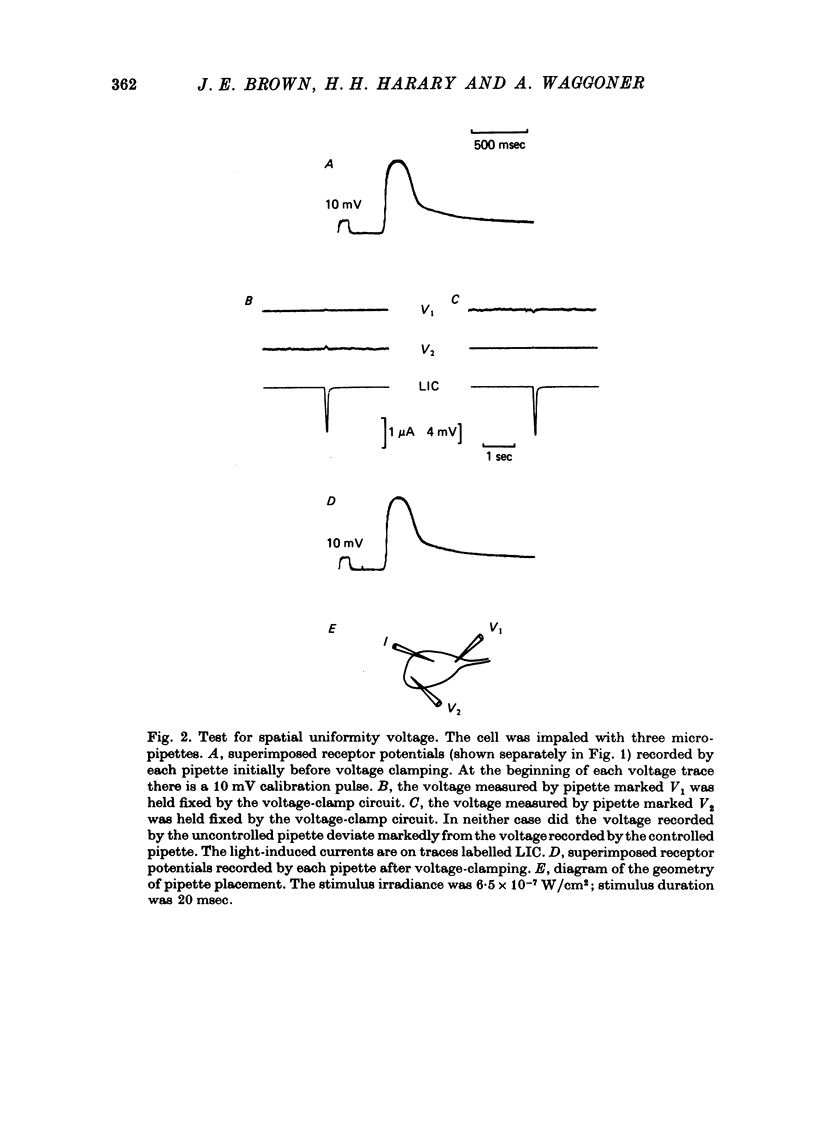
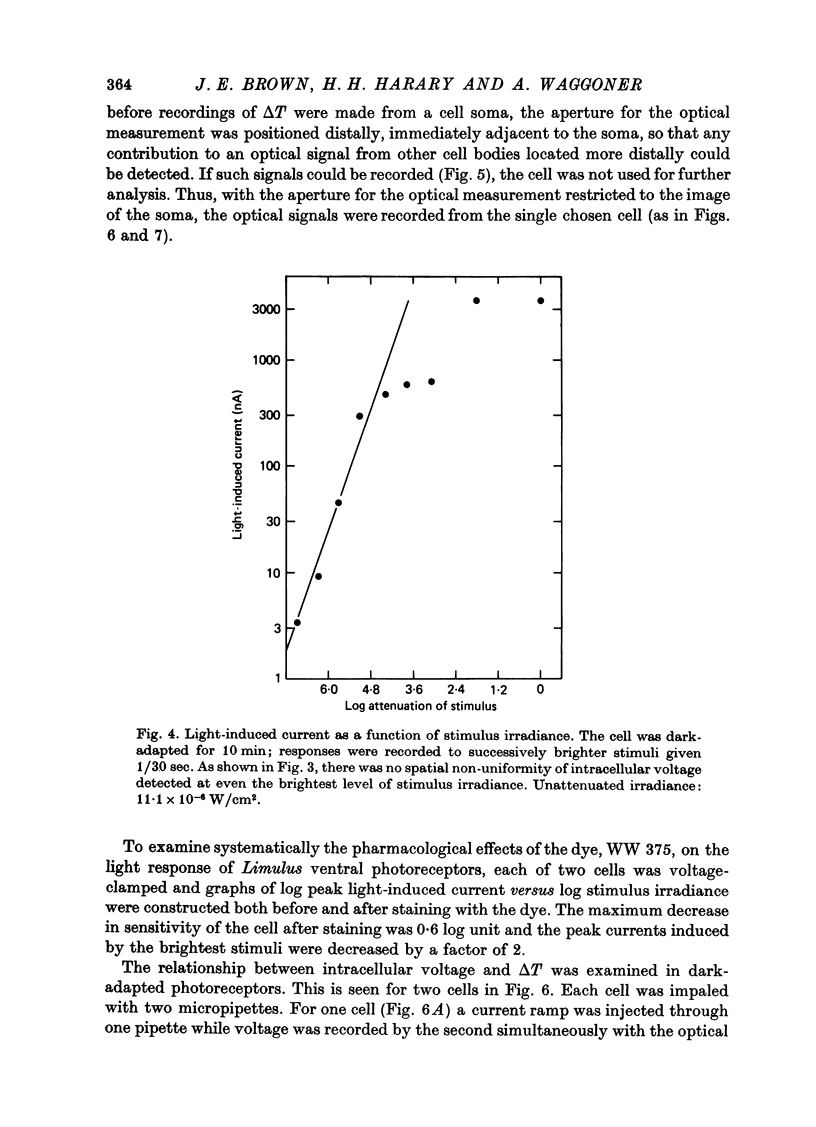
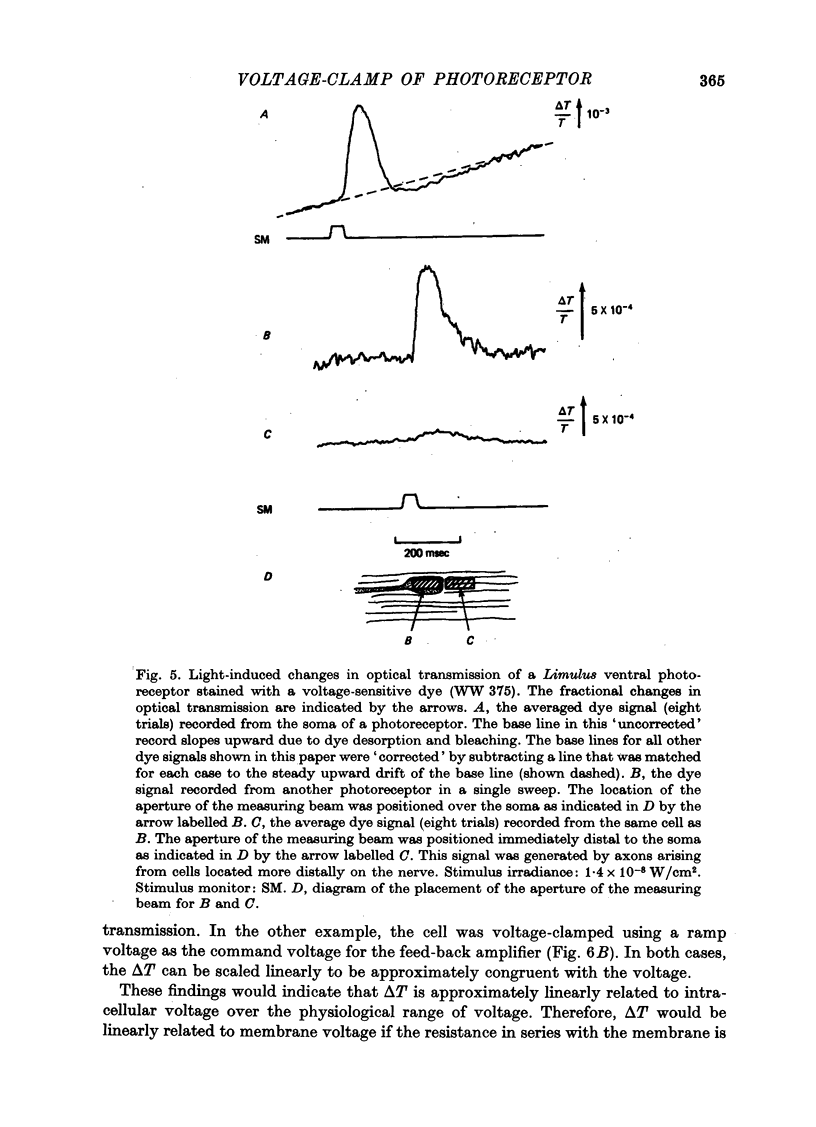
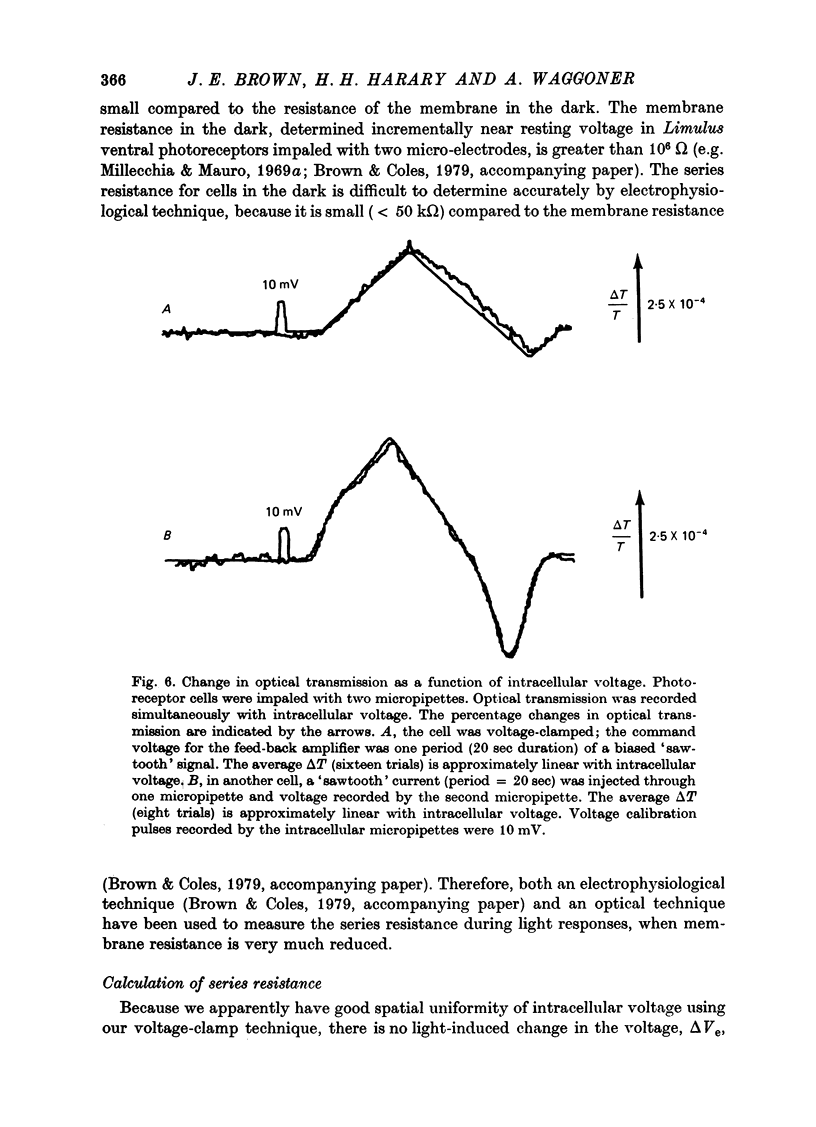
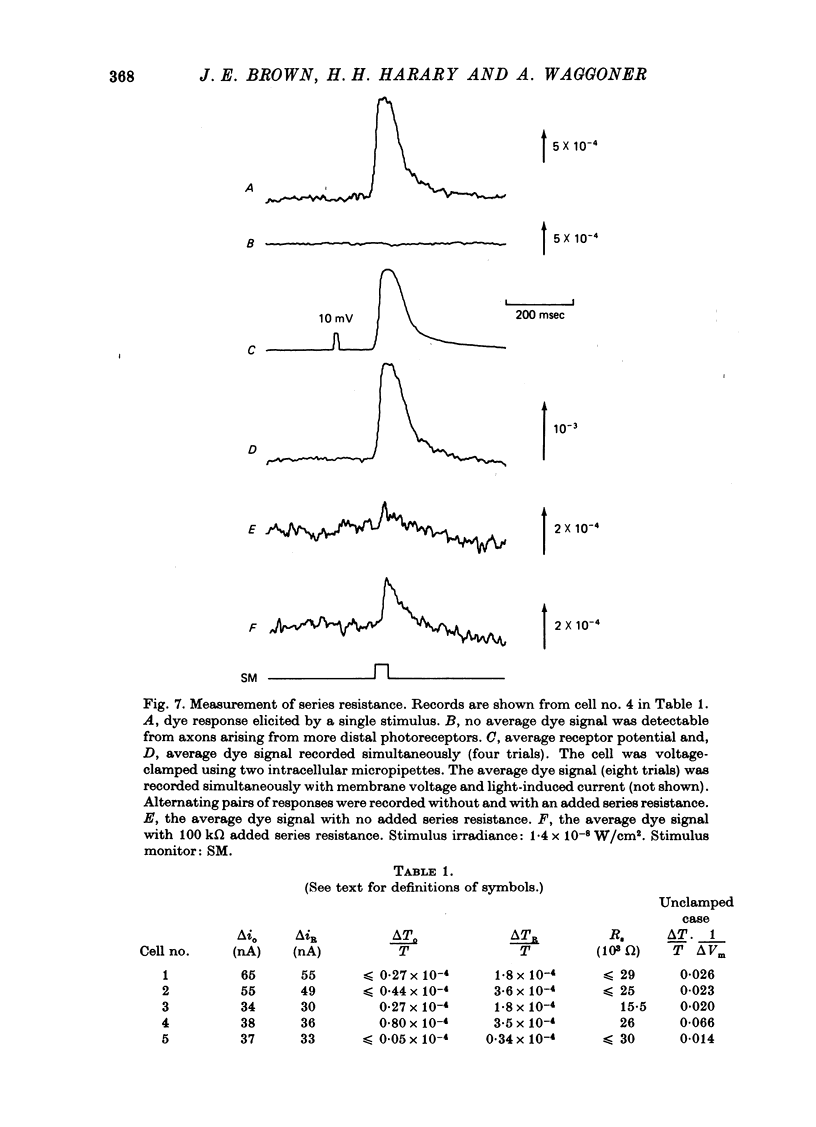
1. Photoreceptor somas in the ventral rudimentary eye of Limulus polyphemus were impaled with three micropipettes. Two micropipettes were connected in a voltage-clamp circuit and the cells were stimulated by brief flashes. The third micropipette did not measure any significant deviations from the 'clamped' voltage during responses to the flashes, in several geometries of electrode placement, even for very bright flashes. Therefore using the described techniques there is no evidence for spatial non-uniformity of intracellular voltage in the soma of these photoreceptors. 2. A voltage-sensitive dye was used to monitor light-induced changes in membrane voltage while intracellular voltage was held clamped by a feed-back circuit. With a known series resistance connected between the bath and ground the dye recorded a light-induced change in membrane voltage. When there was no added series resistance, the light-induced change was smaller and often undetectable. From these data the naturally occurring series resistance was calculated to be less than or equal to 30 k omega. 3. From these measurements, as well as from calculations for a model spherical cell, we conclude that membrane potential can be controlled to within 2 mV using our micropipette 'point clamp' methods, for all but the brightest stimuli.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behbehani M., Srebro R. Discrete waves and phototransduction in voltage-clamped ventral photoreceptors. J Gen Physiol. 1974 Aug;64(2):186–200. [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A. Saturation of the response to light in Limulus ventral photoreceptor. J Physiol. 1979 Nov;296:373–392. doi: 10.1113/jphysiol.1979.sp013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. Intracellular Ca modulates sensitivity and time scale in Limulus ventral photoreceptors. Nature. 1975 Nov 20;258(5532):252–254. doi: 10.1038/258252a0. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. I. The microanatomy. J Gen Physiol. 1969 Sep;54(3):289–309. doi: 10.1085/jgp.54.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Coles J. A., Brown J. E. Effects of increased intracellular pH-buffering capacity on the light response of Limulus ventral photoreceptor. Biochim Biophys Acta. 1976 Jun 4;436(1):140–153. doi: 10.1016/0005-2736(76)90226-1. [DOI] [PubMed] [Google Scholar]
- Davila H. V., Cohen L. B., Salzberg B. M., Shrivastav B. B. Changes in ANS and TNS fluorescence in giant axons from Loligo. J Membr Biol. 1974;15(1):29–46. doi: 10.1007/BF01870080. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Engel E. The spatial variation of membrane potential near a small source of current in a spherical cell. J Gen Physiol. 1970 Jun;55(6):736–757. doi: 10.1085/jgp.55.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Charlton J. S. Enhancement and phototransduction in the ventral eye of limulus. J Gen Physiol. 1977 May;69(5):553–569. doi: 10.1085/jgp.69.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti R., Fuortes M. G. Analysis of responses in visual cells of the leech. J Physiol. 1972 Dec;227(1):173–194. doi: 10.1113/jphysiol.1972.sp010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Brown J. E. Ion fluxes in photoreception in Limulus polyphemus ventral eye. I. The response of potassium efflux to light. Biochim Biophys Acta. 1972 Jul 3;274(1):140–157. doi: 10.1016/0005-2736(72)90289-1. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Poo M. M., Stuart A. E. Passive signal propagation and membrane properties in median photoreceptors of the giant barnacle. J Physiol. 1977 Oct;272(1):25–43. doi: 10.1113/jphysiol.1977.sp012032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Effects of intracellular injection of calcium buffers on light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):489–506. doi: 10.1085/jgp.66.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Light-induced changes of sensitivity in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):473–488. doi: 10.1085/jgp.66.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Decremental conduction of the visual signal in barnacle lateral eye. J Physiol. 1972 Jan;220(1):145–175. doi: 10.1113/jphysiol.1972.sp009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro R., Behbehani M. Light adaptation in the ventral photoreceptor of Limulus. J Gen Physiol. 1974 Aug;64(2):166–185. [PMC free article] [PubMed] [Google Scholar]


