Abstract
1. Limulus ventral photoreceptor cells were voltage-clamped with two intracellular micro-electrodes. The light-induced membrane current was recorded for brief stimuli. From observation of discrete waves (quantum bumps) at low stimulus energies and the early receptor potential at high energies, the stimulus energy was related to the number of rhodopsin molecules photosiomerized. 2. In the dark-adapted cell the log (peak light-induced current) reached almost its maximum value when about 10(3) of the 10(9) rhodopsin molecules in the cell were photoisomerized. 3. The magnitude of the maximum light-induced current was not significantly altered after iontophoresis of EGTA into the cell. This treatment is known to counteract the Ca2+-mediated reduction in sensitivity to light. 4. Current pulses were injected into the unclamped cell during the receptor potential. The form of the voltage deflexion (a step followed by a curve) suggested that the effective electrical equivalent of the cell was a membrane capacitance in parallel with a light-dependent membrane resistance, Rm, and in series with another, light-invariant, resistance, Rs. Rs ranged from 7 to 24 k omega (five cells). 5. During a receptor potential the ratio Rm/Rs was never observed to fall below 1.7 no matter how intense the light flash. Hence, it is concluded that the light-induced current saturated essentially because Rm fell to a minimum value. 6. Charging curves gave a value for the capacitance, and hence the area, of the surface membrane. From this it was estimated that there were 10(5)-10(6) microvilli on each cell. 7. These results show that the light-induced increase in membrane conductance in a dark-adapted cell comes close to its maximum value when the number of photoisomerizations is about 1/1000 the total number of microvilli. We suggest that absorption of a photon by a rhodopsin molecule in a microvillus causes an increase in membrane conductance on parts of the surface membrane beyond that microvillus. 8. In the presence of moderate background illumination the sensitivity to non-saturating superimposed flashes was greatly decreased (e.g. by 10(3) while the saturating light-induced current was only slightly decreased (e.g. by 15%). At higher background intensities the saturating light-induced current was further decreased (e.g., with a background that photoisomerized 10(6.25) molecules per second the saturating light-induced current was reduced by 47%).
Full text
PDF


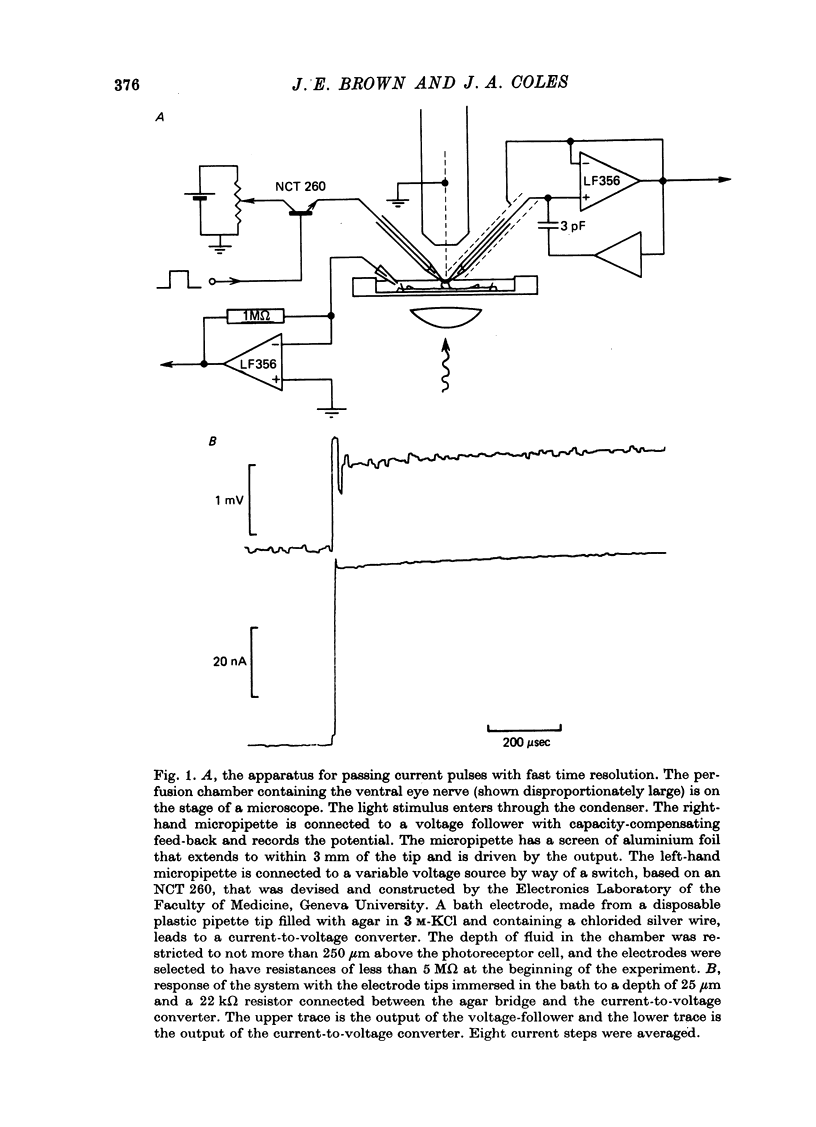
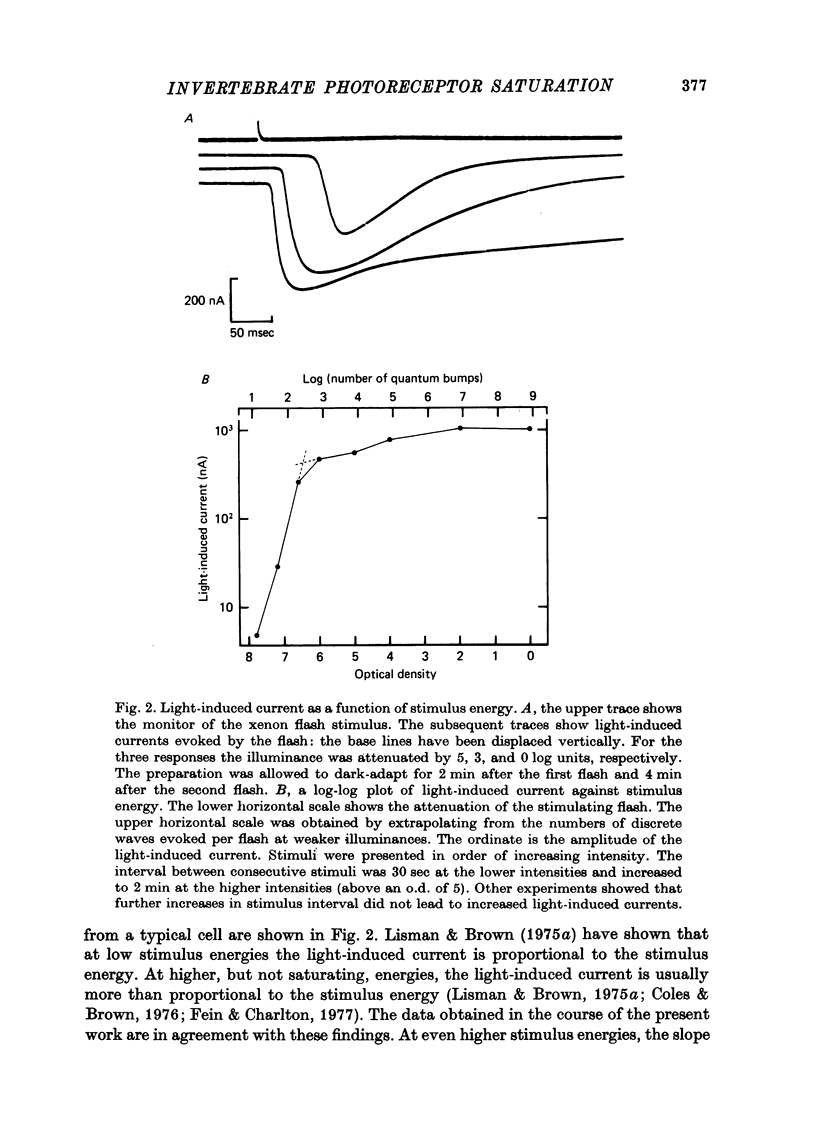

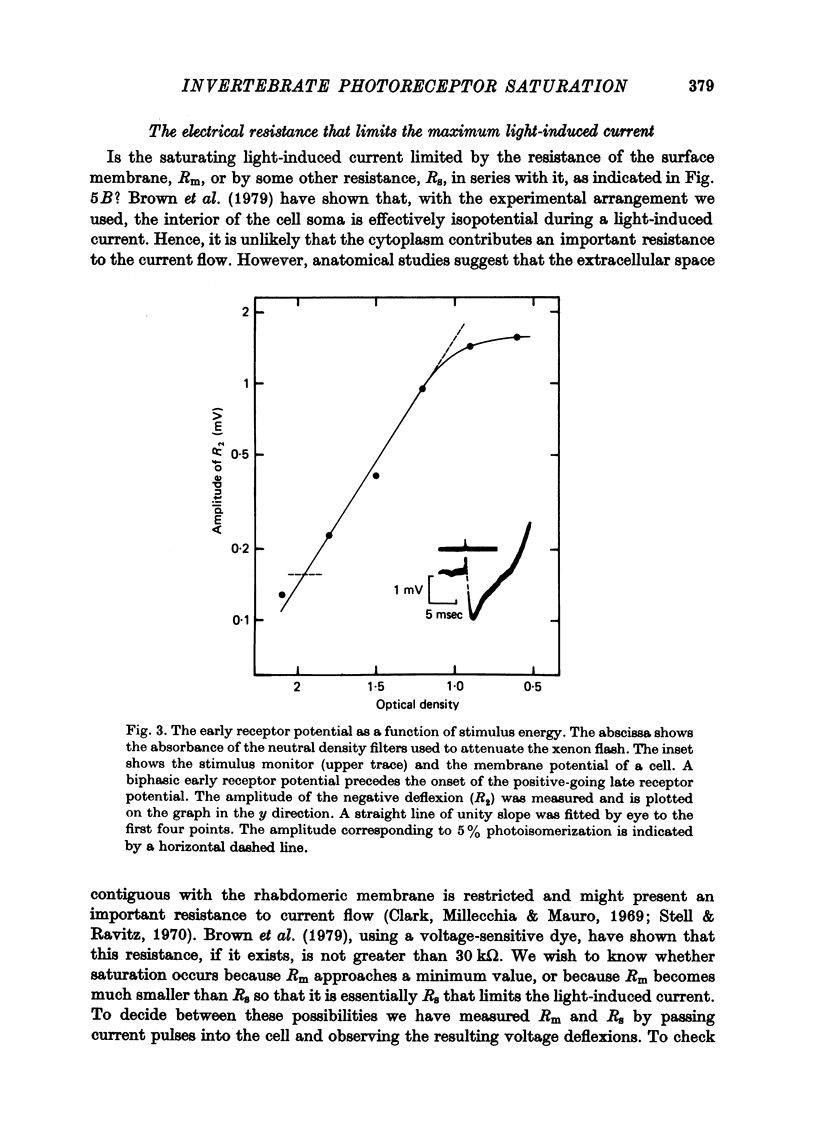

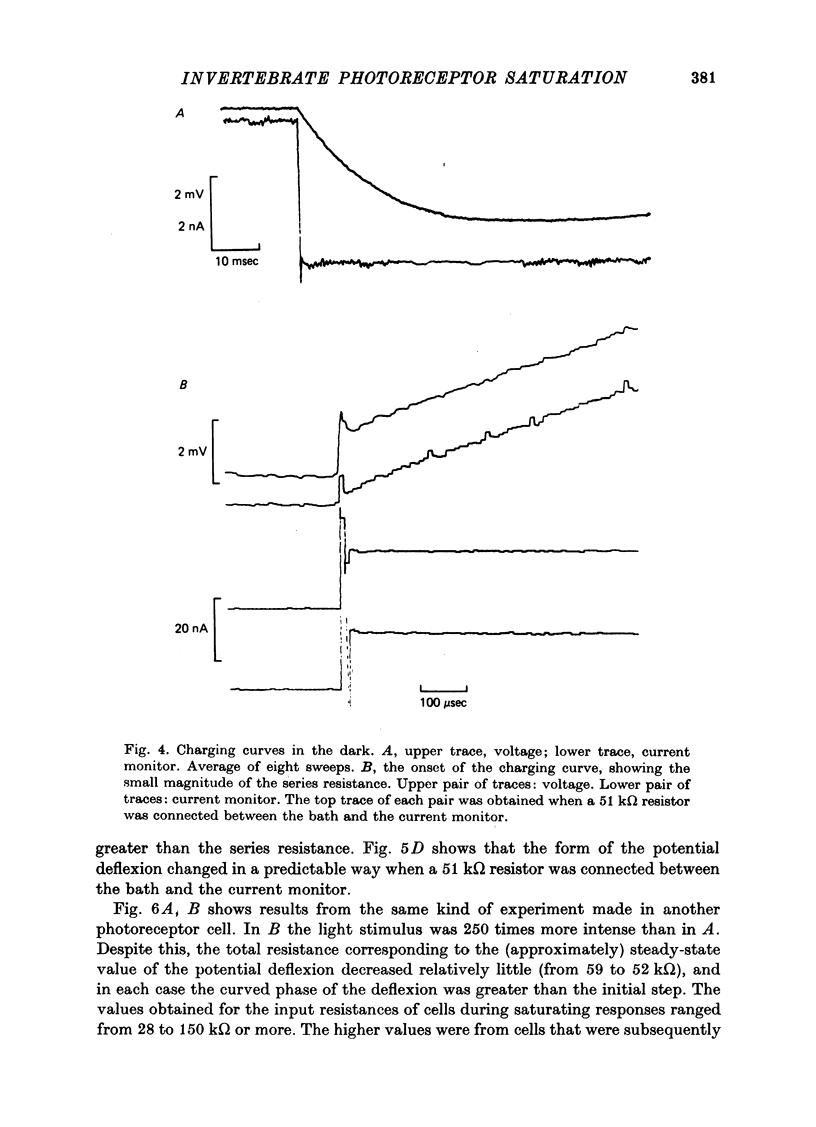
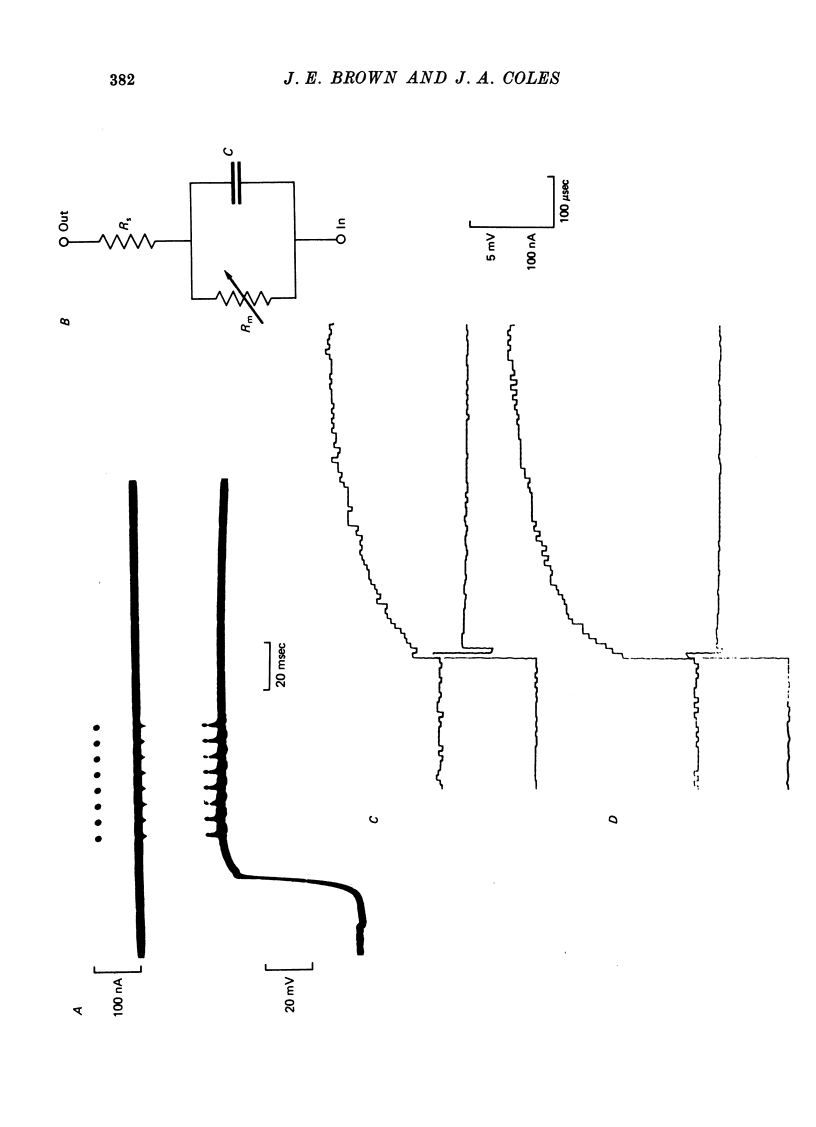
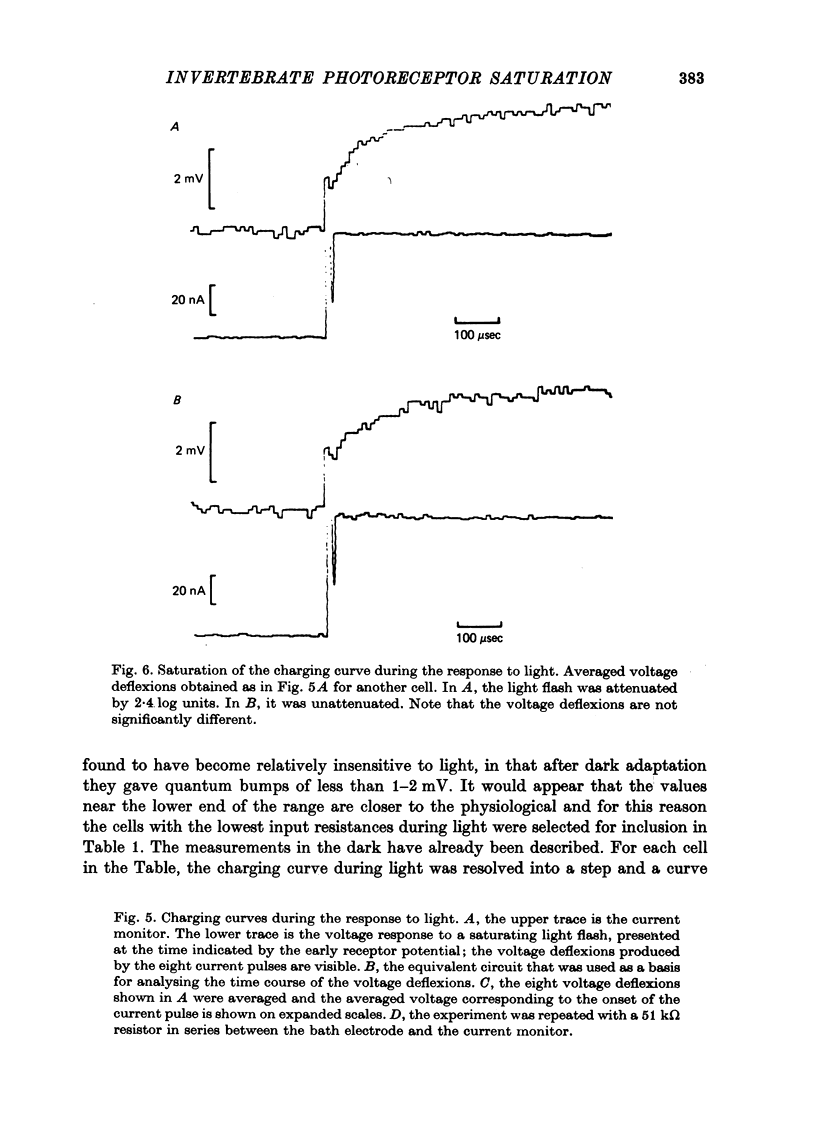
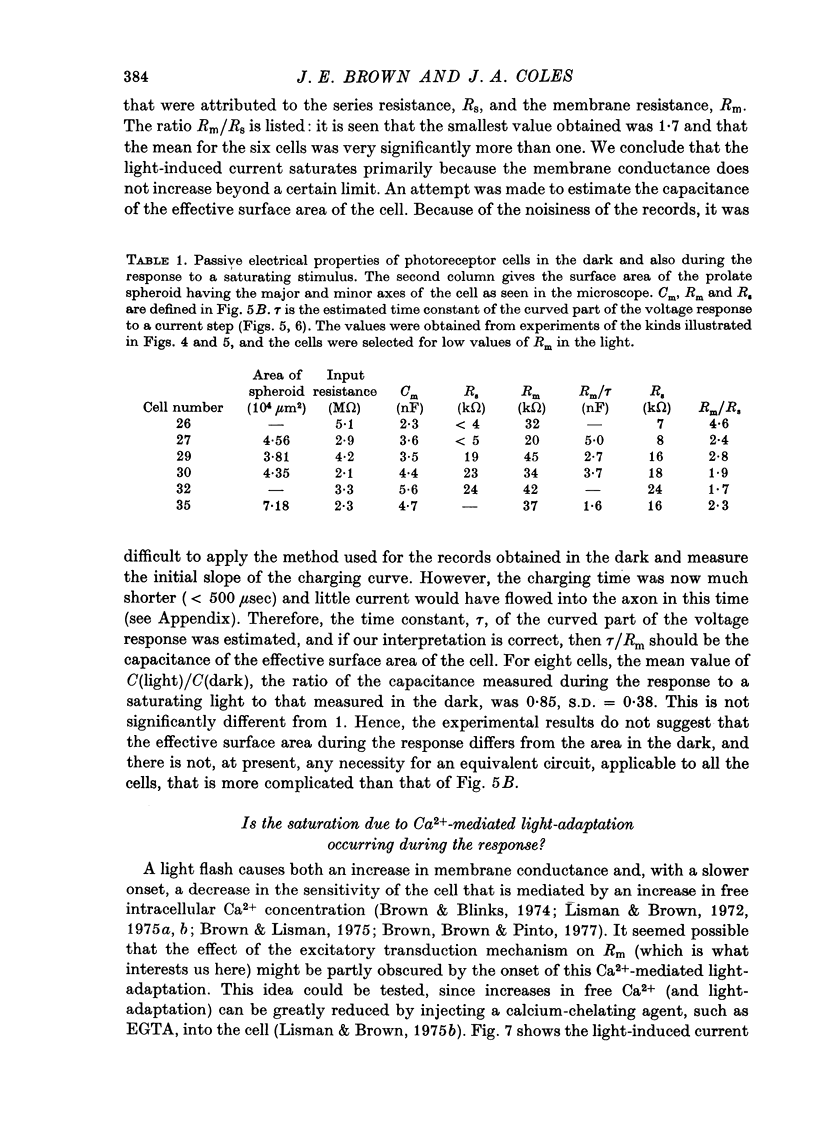
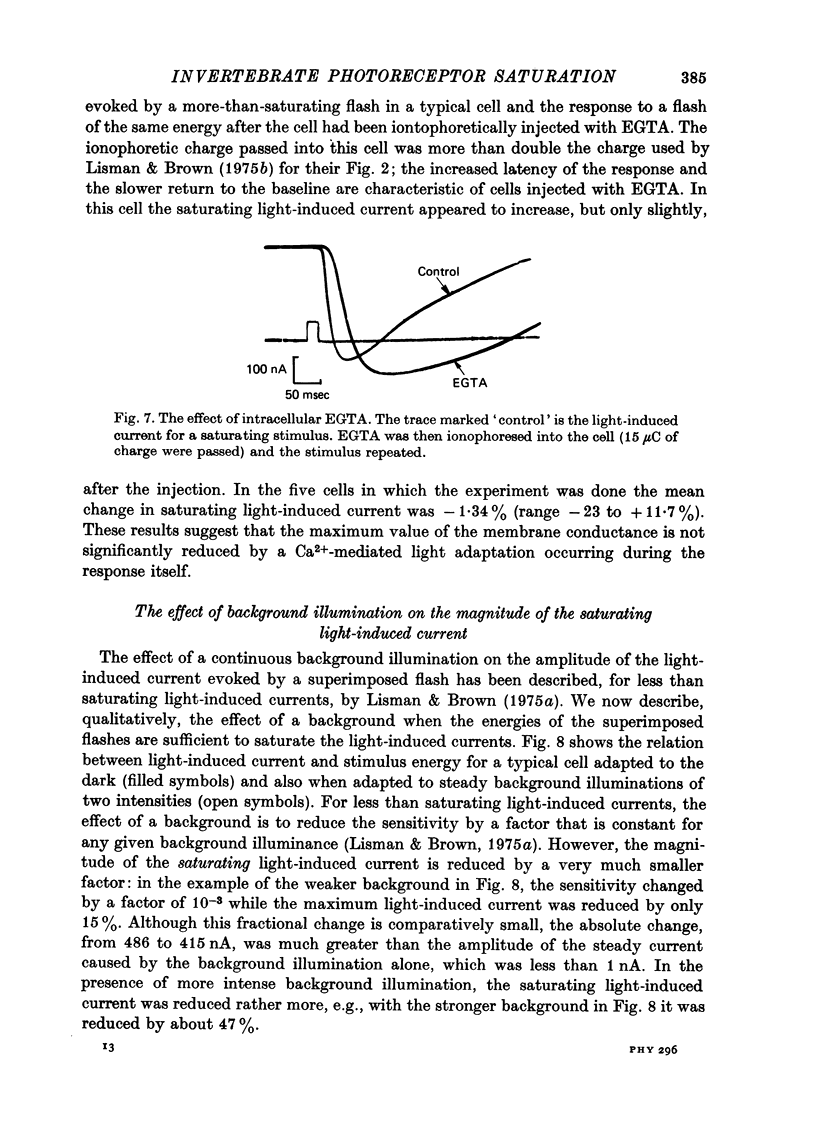
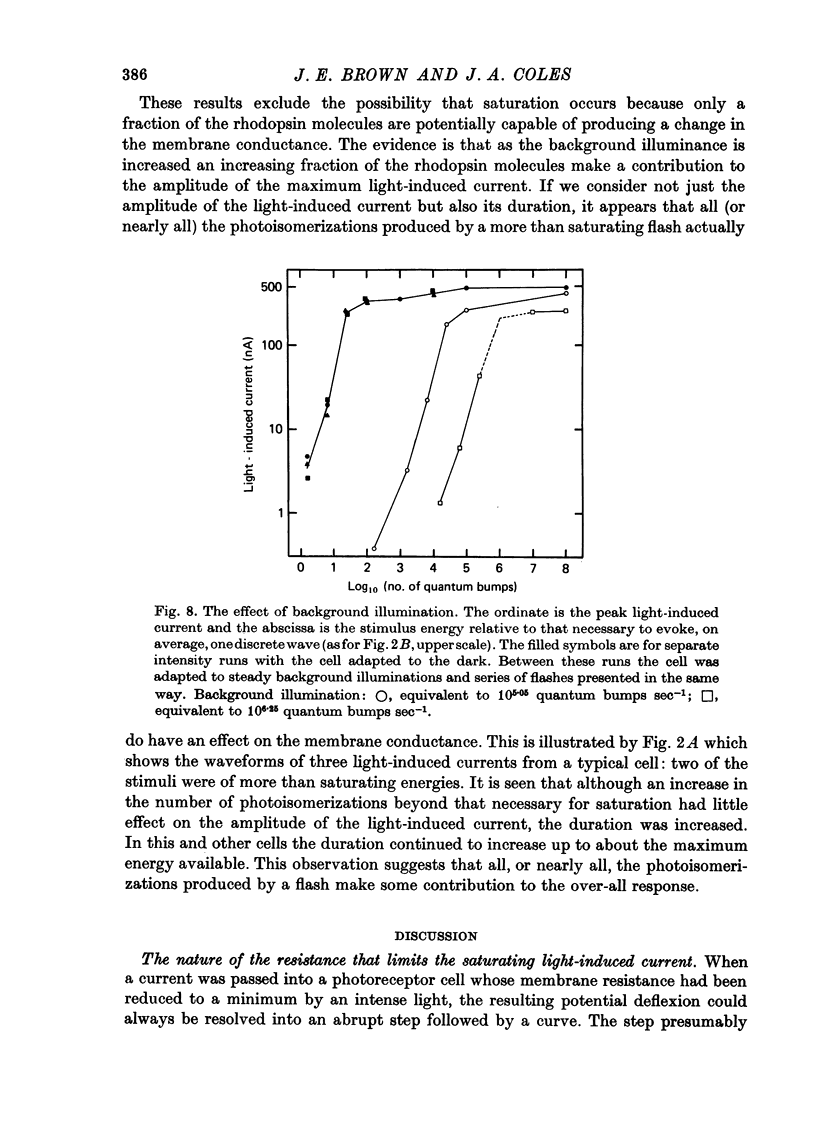



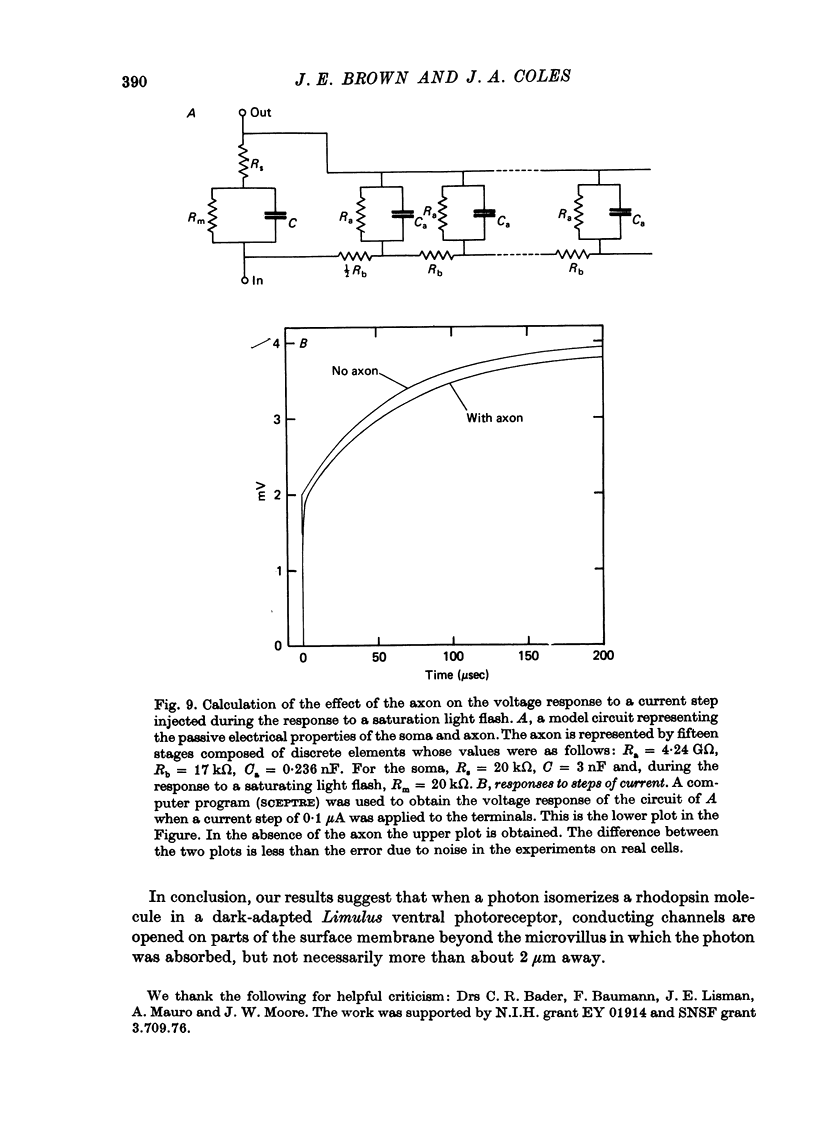


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Brown P. K., Pinto L. H. Detection of light-induced changes of intracellular ionized calcium concentration in Limulus ventral photoreceptors using arsenazo III. J Physiol. 1977 May;267(2):299–320. doi: 10.1113/jphysiol.1977.sp011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Harary H. H., Waggoner A. Isopotentiality and an optical determination of series resistance in Limulus ventral photoreceptors. J Physiol. 1979 Nov;296:357–372. doi: 10.1113/jphysiol.1979.sp013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. Intracellular Ca modulates sensitivity and time scale in Limulus ventral photoreceptors. Nature. 1975 Nov 20;258(5532):252–254. doi: 10.1038/258252a0. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. I. The microanatomy. J Gen Physiol. 1969 Sep;54(3):289–309. doi: 10.1085/jgp.54.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S., Hodgkin A. L. MEMBRANE AND PROTOPLASM RESISTANCE IN THE SQUID GIANT AXON. J Gen Physiol. 1939 May 20;22(5):671–687. doi: 10.1085/jgp.22.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Brown J. E. Effects of increased intracellular pH-buffering capacity on the light response of Limulus ventral photoreceptor. Biochim Biophys Acta. 1976 Jun 4;436(1):140–153. doi: 10.1016/0005-2736(76)90226-1. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., YEANDLE S. PROBABILITY OF OCCURRENCE OF DISCRETE POTENTIAL WAVES IN THE EYE OF LIMULUS. J Gen Physiol. 1964 Jan;47:443–463. doi: 10.1085/jgp.47.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Charlton J. S. Enhancement and phototransduction in the ventral eye of limulus. J Gen Physiol. 1977 May;69(5):553–569. doi: 10.1085/jgp.69.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. R., Nickel E. E. Ultrastructural and molecular characteristics of crayfish photoreceptor membranes. J Cell Biol. 1976 Jun;69(3):721–732. doi: 10.1083/jcb.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti R., Fuortes M. G. Analysis of responses in visual cells of the leech. J Physiol. 1972 Dec;227(1):173–194. doi: 10.1113/jphysiol.1972.sp010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Mirolli M. The passive electrical properties of the membrane of a molluscan neurone. J Physiol. 1972 Dec;227(1):35–49. doi: 10.1113/jphysiol.1972.sp010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGINS W. A., ZONANA H. V., ADAMS R. G. Local membrane current in the outer segments of squid photoreceptors. Nature. 1962 Jun 2;194:844–847. doi: 10.1038/194844a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Bering H. Electrophysiological measurement of the number of rhodopsin molecules in single Limulus photoreceptors. J Gen Physiol. 1977 Nov;70(5):621–633. doi: 10.1085/jgp.70.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Effects of intracellular injection of calcium buffers on light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):489–506. doi: 10.1085/jgp.66.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Light-induced changes of sensitivity in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):473–488. doi: 10.1085/jgp.66.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Two light-induced processes in the photoreceptor cells of Limulus ventral eye. J Gen Physiol. 1971 Nov;58(5):544–561. doi: 10.1085/jgp.58.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Sheline Y. Analysis of the rhodopsin cycle in limulus ventral photoreceptors using the early receptor potential. J Gen Physiol. 1976 Nov;68(5):487–501. doi: 10.1085/jgp.68.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose J. S., Lisman J. E. Voltage-sensitive potassium channels in Limulus ventral photoreceptors. J Gen Physiol. 1978 Jan;71(1):101–120. doi: 10.1085/jgp.71.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. R. Decremental conduction of the visual signal in barnacle lateral eye. J Physiol. 1972 Jan;220(1):145–175. doi: 10.1113/jphysiol.1972.sp009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS T. P. PHOTOREVERSAL OF RHODOPSIN BLEACHING. J Gen Physiol. 1964 Mar;47:679–689. doi: 10.1085/jgp.47.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F. Nature of light-induced conductance changes in ventral photoreceptors of Limulus. Nature. 1978 Nov 2;276(5683):76–79. doi: 10.1038/276076a0. [DOI] [PubMed] [Google Scholar]
- Yeandle S., Spiegler J. B. Light-evoked and spontaneous discrete waves in the ventral nerve photoreceptor of Limulus. J Gen Physiol. 1973 May;61(5):552–571. doi: 10.1085/jgp.61.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]


