Abstract
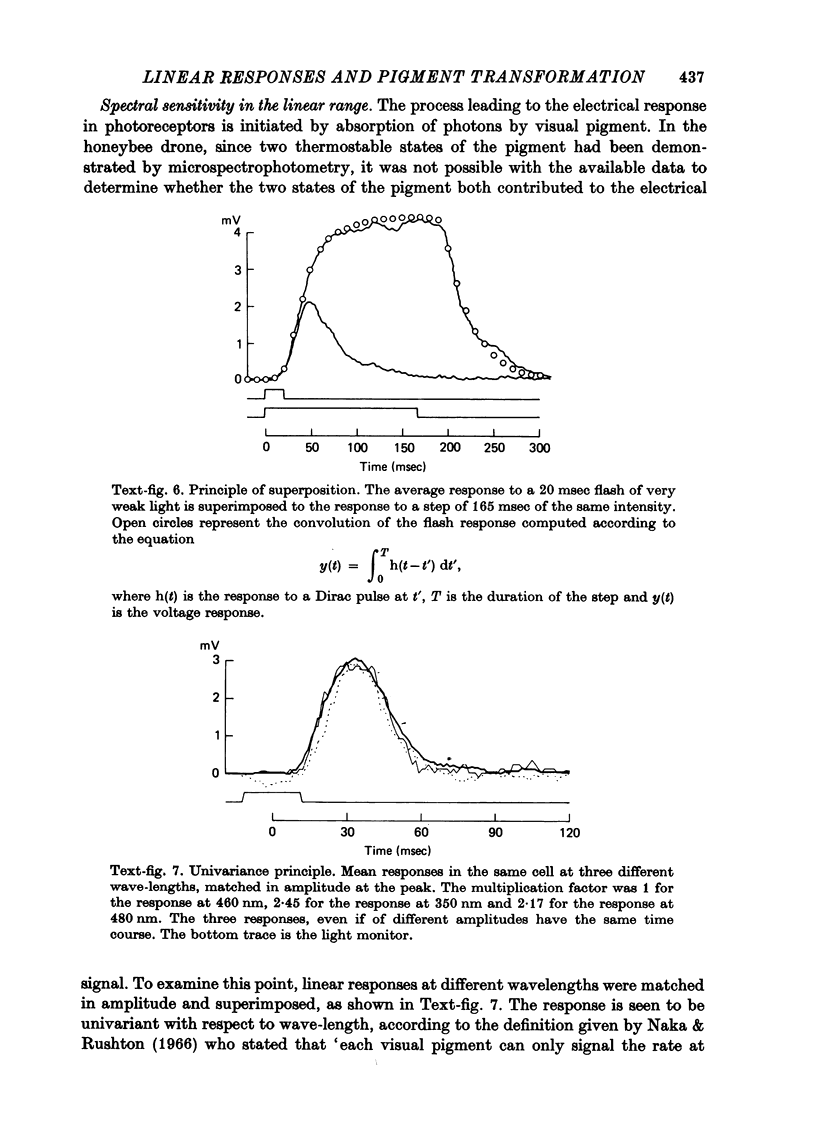
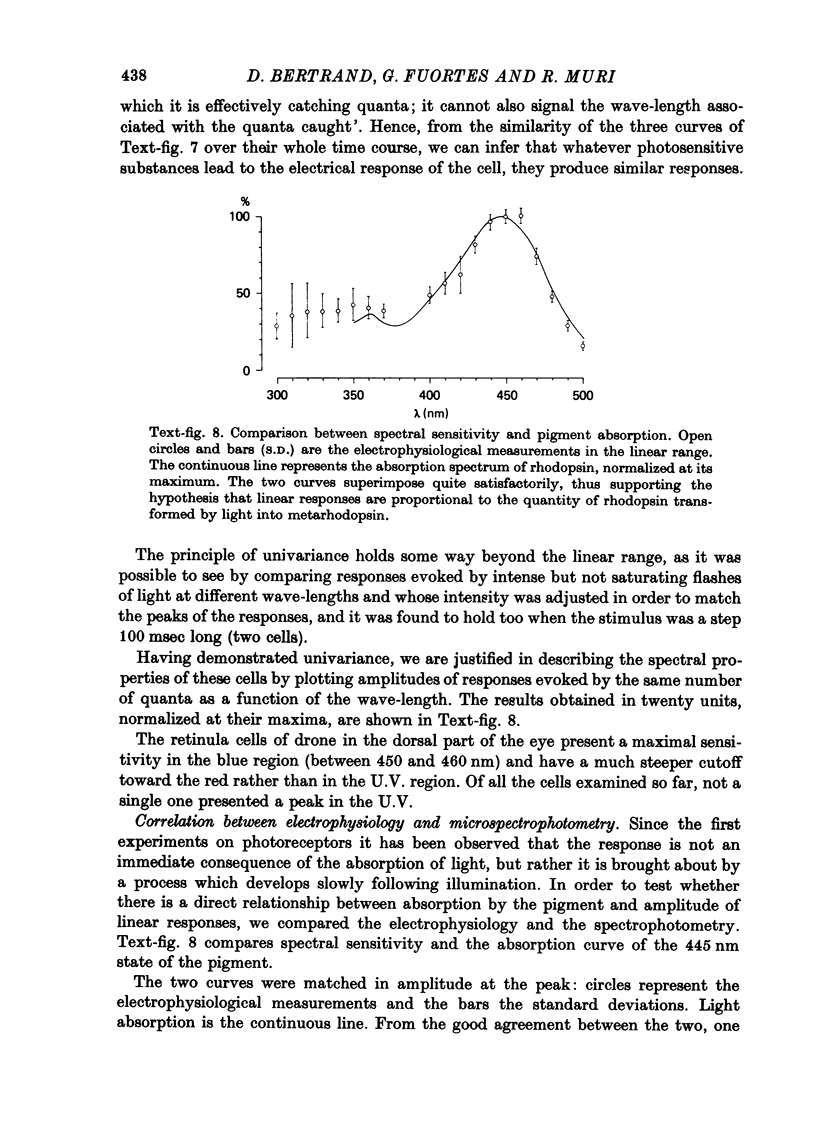
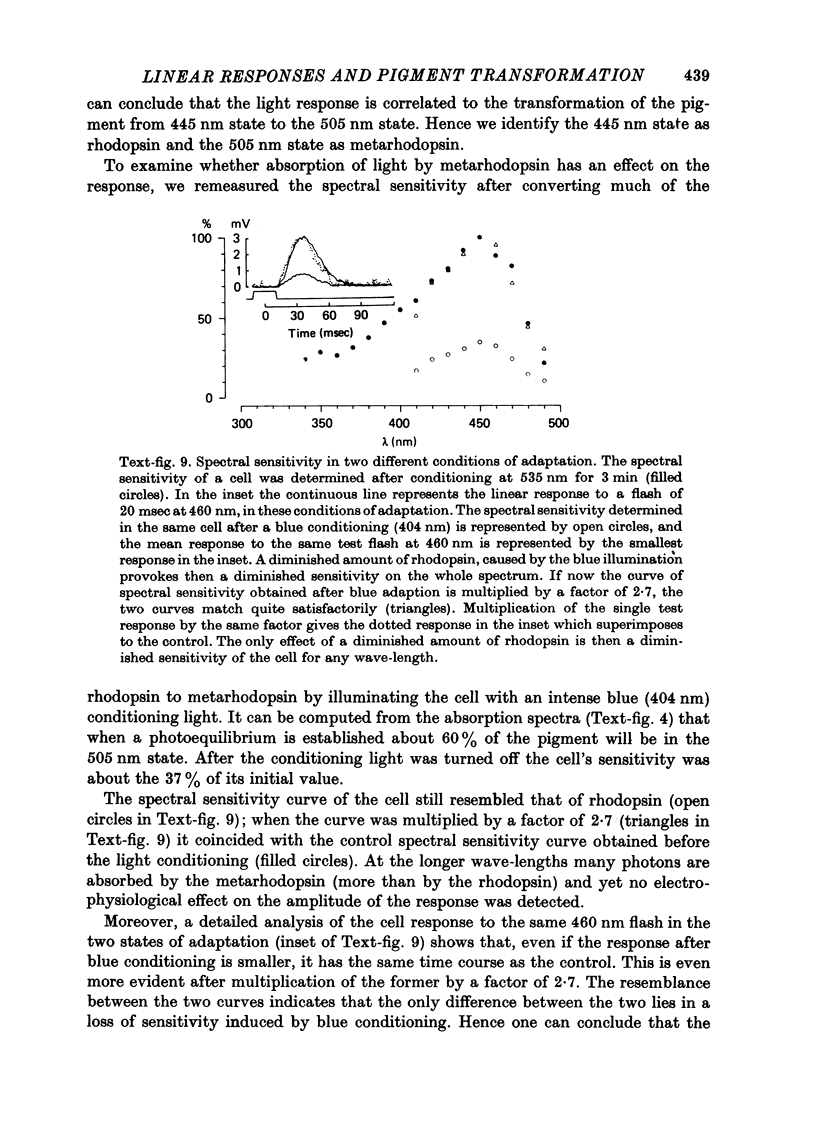
1. Receptor potentials in honeybee drone retinula cells were recorded with intracellular micro-electrodes in the dorsal part of the superfused retina. The light stimuli were sufficiently weak that the response amplitude was proportional to the intensity. 2. Responses to stimuli of different wave-lengths, although of different amplitude, all had the same time course. 3. The maximal sensitivity in all the cells recorded from was to a wave-length between 450 and 460 nm. 4. Microspectrophotometry showed the presence of a pigment with two stable states, interconvertible by light, absorbing maximally at 445 nm (rhodopsin) and 505 nm (metarhodopsin). 5. There was a good match between the absorption spectrum of rhodopsin and the spectral sensitivity of retinula cells. 6. Transformation of a large fraction of rhodopsin to metarhodopsin by light reduced the sensitivity of the retinula cell but did not alter the shape of the relative spectral sensitivity curve or the time course of the responses. 7. It is concluded that for weak lights the receptor potential is determined only by the number of rhodopsin molecules that absorb photons: neither the presence of metarhodopsin nor its phototransformation to rhodopsin produces a detectable effect.
Full text
PDF

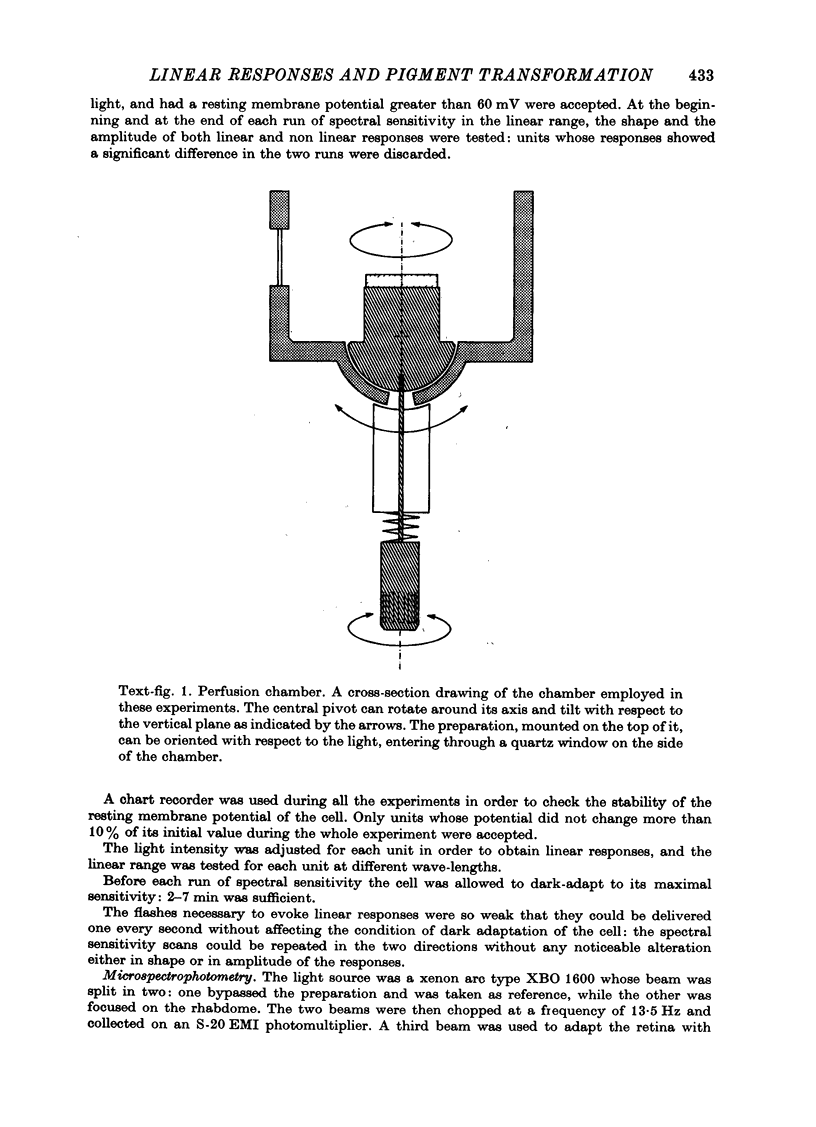
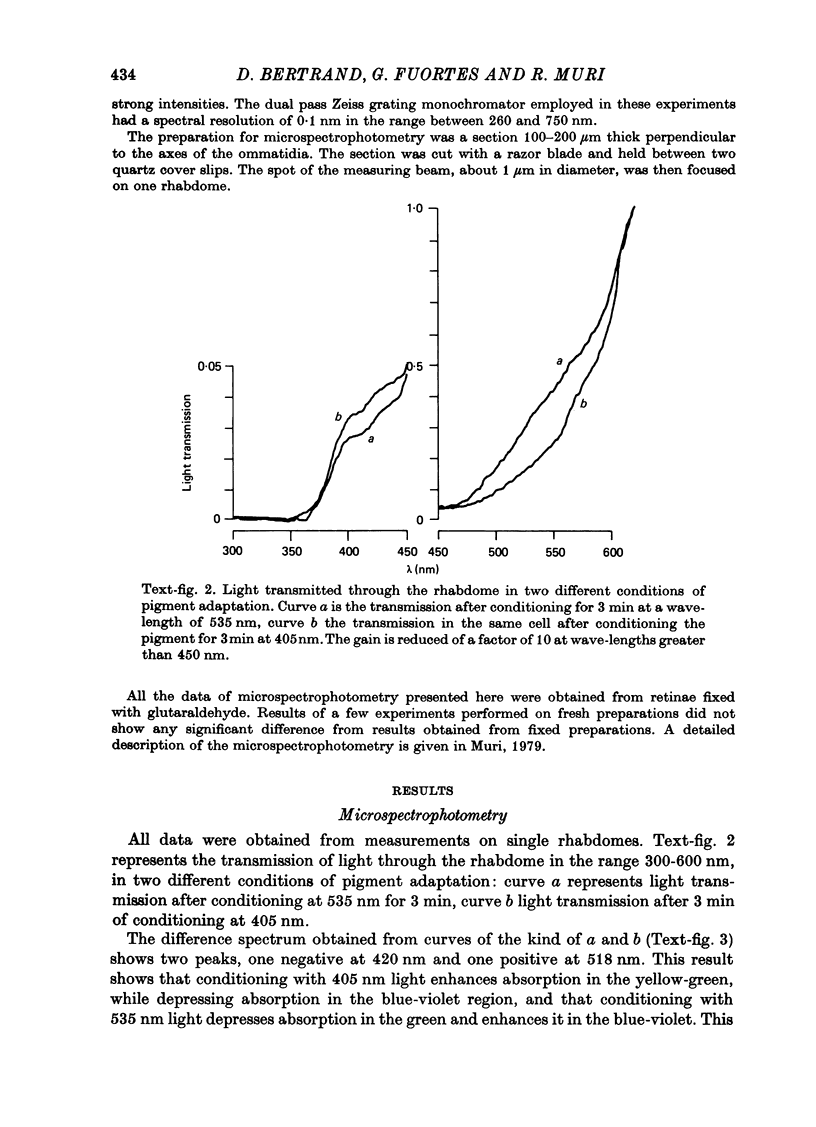
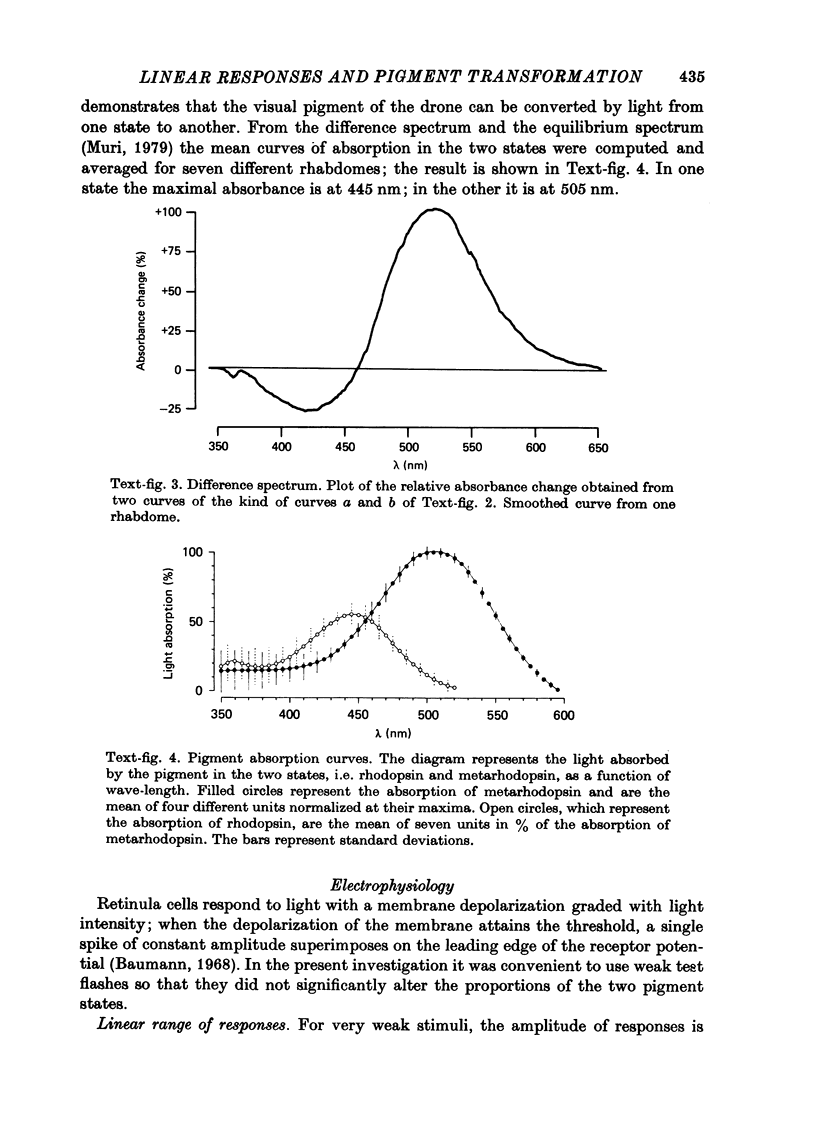
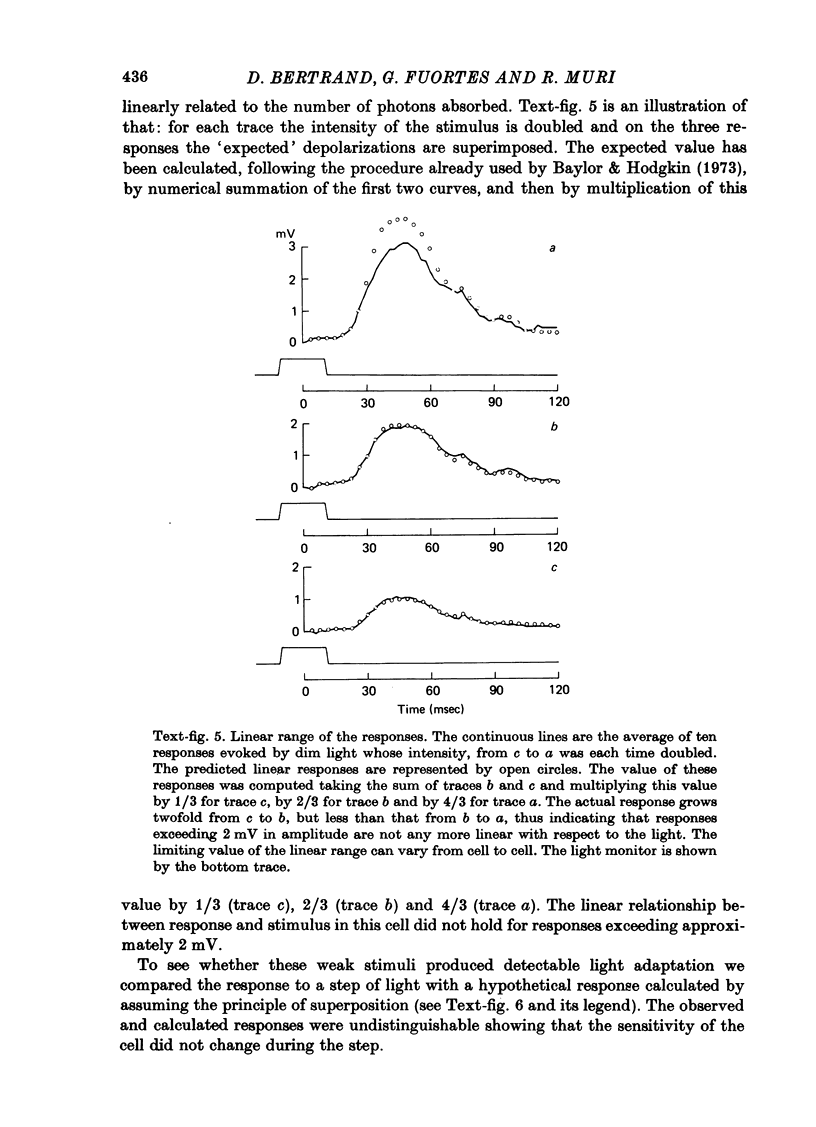



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN P. K., BROWN P. S. Visual pigments of the octopus and cuttlefish. Nature. 1958 Nov 8;182(4645):1288–1290. doi: 10.1038/1821288a0. [DOI] [PubMed] [Google Scholar]
- Bader C., Baumann F., Bertrand D. Role of intracellular calcium and sodium in light adaptation in the retina of the honey bee drone (Apis mellifera, L). J Gen Physiol. 1976 Apr;67(4):475–491. doi: 10.1085/jgp.67.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann F., Hadjilazaro B. A depolarizing aftereffect of intense light in the drone visual receptor. Vision Res. 1972 Jan;12(1):17–31. doi: 10.1016/0042-6989(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Baumann F. Slow and spike potentials recorded from retinula cells of the honeybee drone in response to light. J Gen Physiol. 1968 Dec;52(6):855–875. doi: 10.1085/jgp.52.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD R., ST GEORGE R. C. The rhodopsin system of the squid. J Gen Physiol. 1958 Jan 20;41(3):501–528. doi: 10.1085/jgp.41.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Minke B., Hillman P. Antagonistic components of the late receptor potential in the barnacle photoreceptor arising from different stages of the pigment process. J Gen Physiol. 1973 Jul;62(1):105–128. doi: 10.1085/jgp.62.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- Minke B., Hochstein S., Hillman P. Letter: Antagonistic process as source of visible-light suppression of afterpotential in Limulus UV photoreceptors. J Gen Physiol. 1973 Dec;62(6):787–791. doi: 10.1085/jgp.62.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. Electrophysiological properties of cells in the median ocellus of Limulus. J Gen Physiol. 1972 Feb;59(2):167–185. doi: 10.1085/jgp.59.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. Ultraviolet-induced sensitivity to visible light in ultraviolet receptors of Limulus. J Gen Physiol. 1972 Feb;59(2):186–200. doi: 10.1085/jgp.59.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]



