Abstract
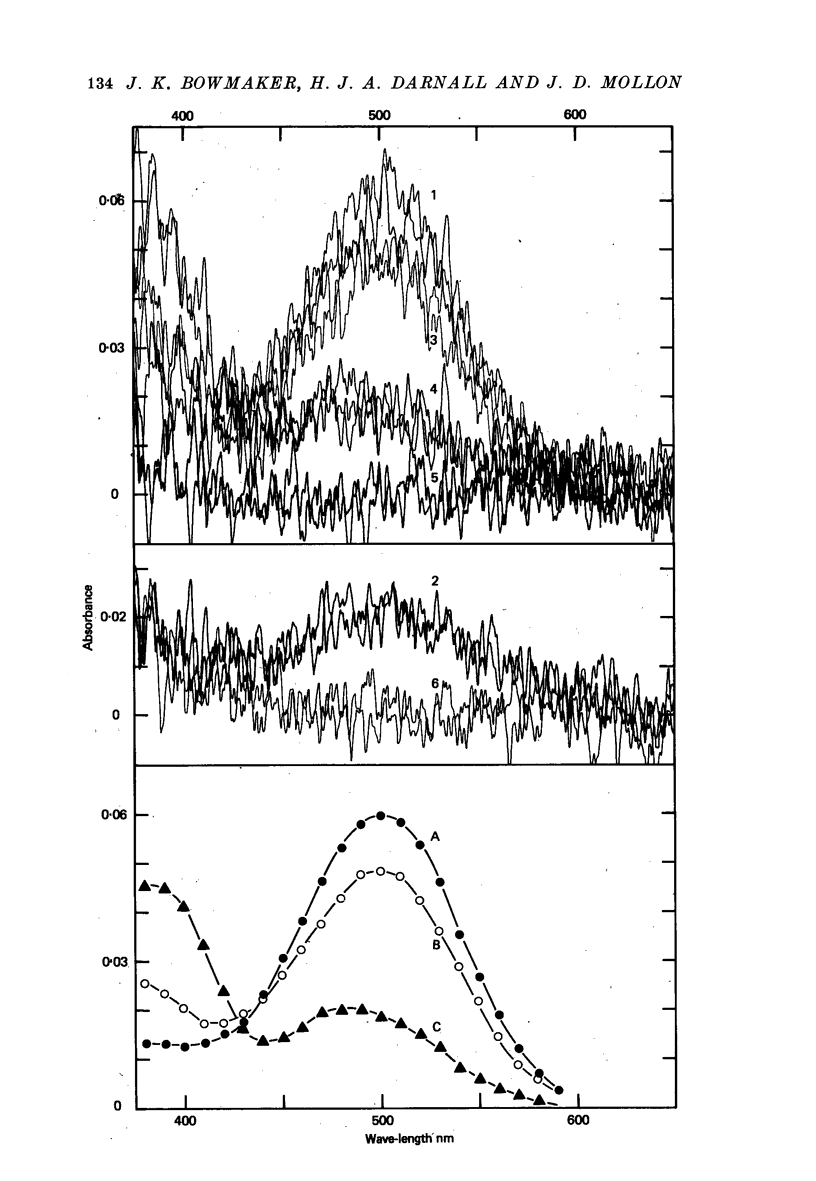
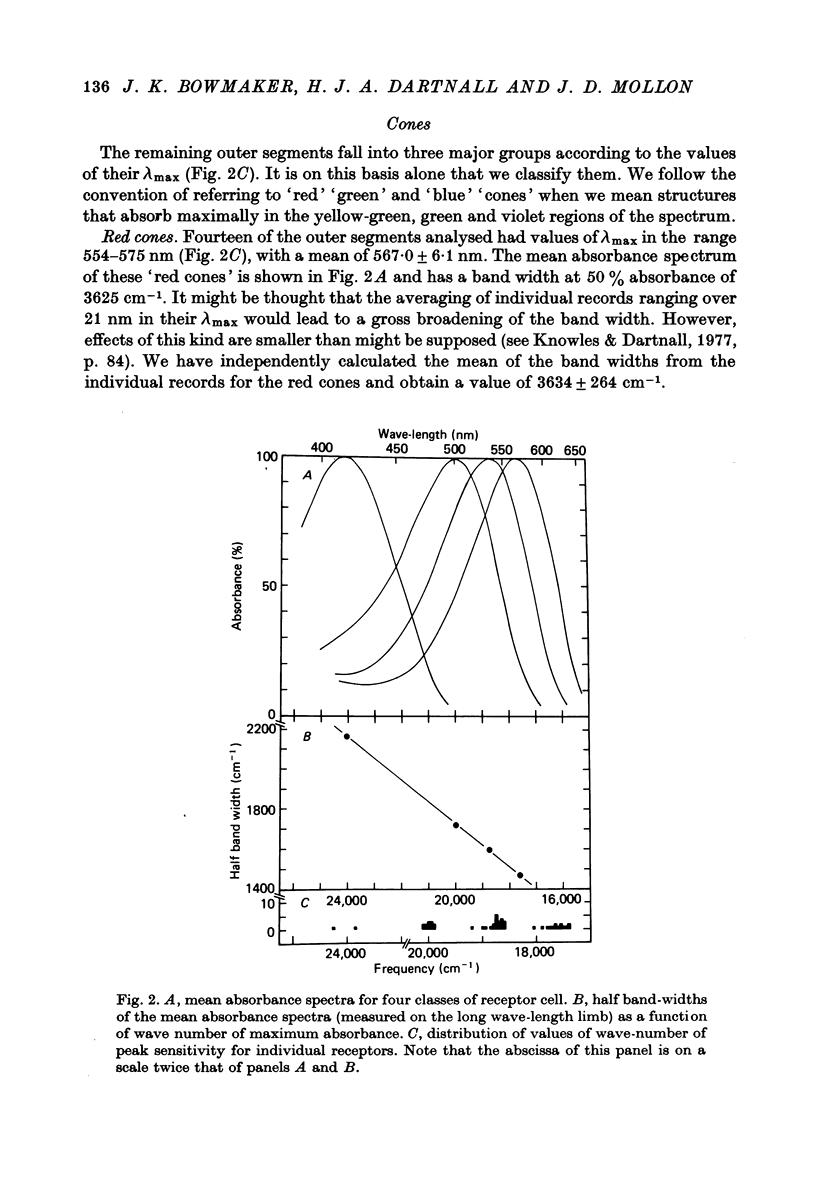
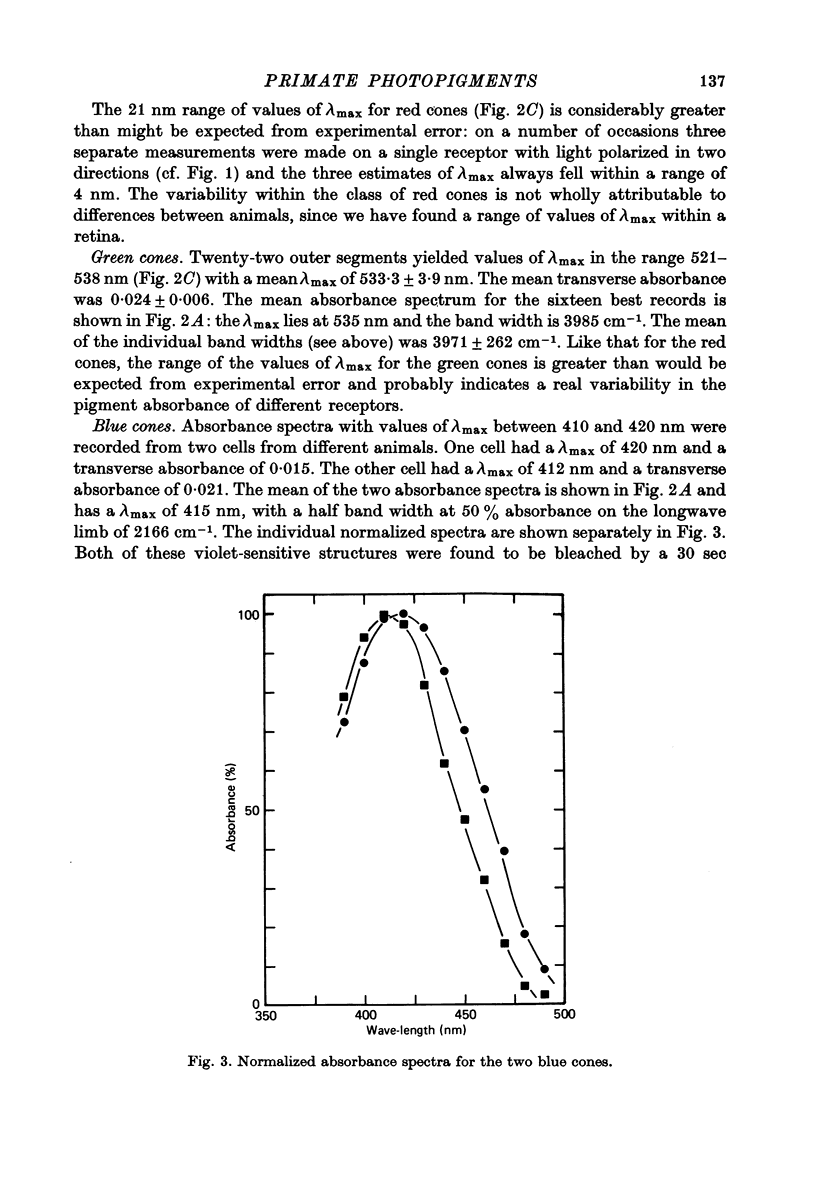
1. Microspectrophotometric measurements reveal four classes of photoreceptor in the retina of the cynomolgus monkey, Macaca fascicularis, which is known to possess colour vision similar to that of a normal human trichromat. 2. Although the eyes were removed in bright illumination, the densities of pigment were comparable to those we have measured in dark-adapted rhesus retinae. 3. The mean wave-lengths of peak sensitivity (lambda max) for the four classes of photoreceptor were 415, 500, 535 and 567 nm. 4. The band widths of the absorbance spectra decreased linearly as the wave-number of peak sensitivity decreased. 5. If, by assuming a reasonable value for the axial density of the rod outer segment and correcting for lens absorption, a spectral sensitivity for human vision is reconstructed from the P500 pigment, it is found to be systematically broader than the CIE scotopic sensitivity function. 6. Given explicit assumptions, it is possible from the P535 and P567 pigments to reconstruct human psychophysical sensitivities that resemble the pi 4 and pi 5 mechanisms of W. S. Stiles. 7. Although the P415 pigment has a lambda max much shorter than that of the psychophysically measured blue mechanisms, the two spectral-sensitivity functions are brought into proximity when the microspectrophotometric data are corrected for absorption by the optic media.
Full text
PDF





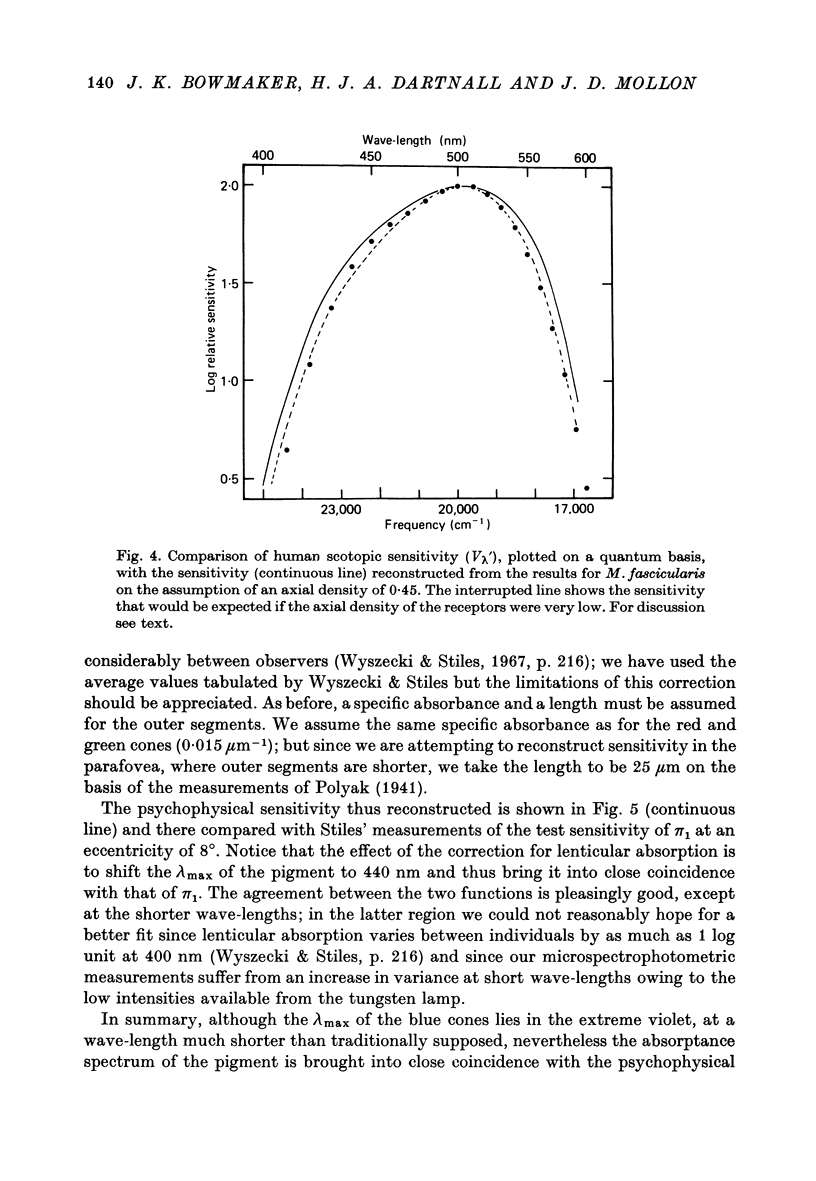
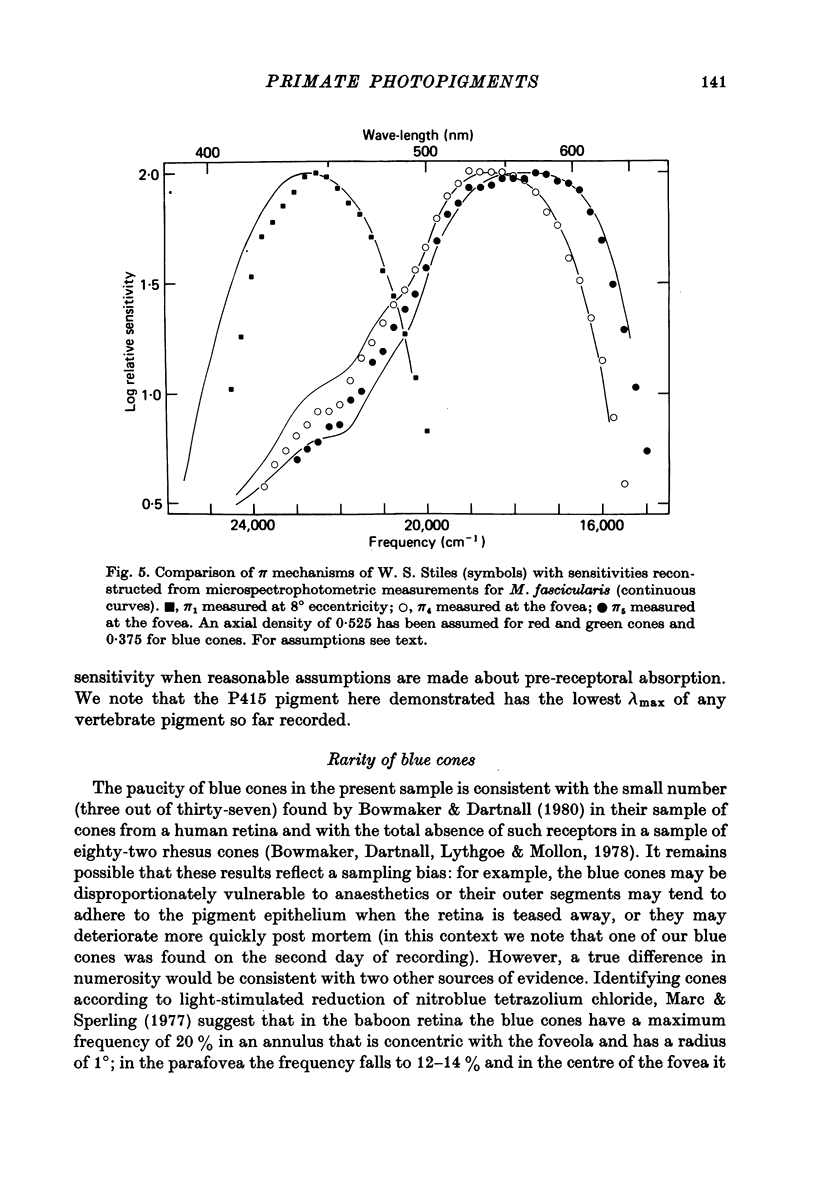


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINDLEY G. S. The summation areas of human colour-receptive mechanisms at increment threshold. J Physiol. 1954 May 28;124(2):400–408. doi: 10.1113/jphysiol.1954.sp005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J., Lythgoe J. N., Mollon J. D. The visual pigments of rods and cones in the rhesus monkey, Macaca mulatta. J Physiol. 1978 Jan;274:329–348. doi: 10.1113/jphysiol.1978.sp012151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Loew E. R., Liebman P. A. Variation in the lambdamax of rhodopsin from individual frogs. Vision Res. 1975 Aug-Sep;15:997–1003. doi: 10.1016/0042-6989(75)90242-4. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. Long-lived photoproducts of the green-rod pigment of the frog, Rana temporaria. Vision Res. 1977;17(1):17–23. doi: 10.1016/0042-6989(77)90195-x. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 1977;17(10):1129–1138. doi: 10.1016/0042-6989(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Bridges C. D. Spectroscopic properties of porphyropsins. Vision Res. 1967 May;7(5):349–369. doi: 10.1016/0042-6989(67)90044-2. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Estévez O., Cavonius C. R. Human color perception and Stiles' pi mechanisms. Vision Res. 1977;17(3):417–422. doi: 10.1016/0042-6989(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Sensitive low-light-level microspectrophotometer: detection of photosensitive pigments of retinal cones. J Opt Soc Am. 1964 Dec;54(12):1451–1459. doi: 10.1364/josa.54.001451. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Sperling H. G. Chromatic organization of primate cones. Science. 1977 Apr 22;196(4288):454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Sigel C. Evaluation of the candidacy of the pi-mechanisms of Stiles for color-matching fundamentals. Vision Res. 1978;18(3):317–330. doi: 10.1016/0042-6989(78)90166-9. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Henry G. H. Bleaching and regeneration of cone pigments in man. Vision Res. 1968 Jun;8(6):617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., SMITH P. H. Iodopsin. J Gen Physiol. 1955 May 20;38(5):623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Blue-blindness in the normal fovea. J Opt Soc Am. 1967 Nov;57(11):1289–1301. doi: 10.1364/josa.57.001289. [DOI] [PubMed] [Google Scholar]