Abstract
1. The effects of efferent electric pelvic nerve stimulation on colorectal motility and blood flow with emphasis on the motor responses in consecutive colonic and rectal segments were studied in anaesthetized cats. It was considered of particular interest to explore whether selective pharmacological blockade and graded nerve stimulations might reveal the presence of functionally differentiated efferent fibres controlling colonic motility.
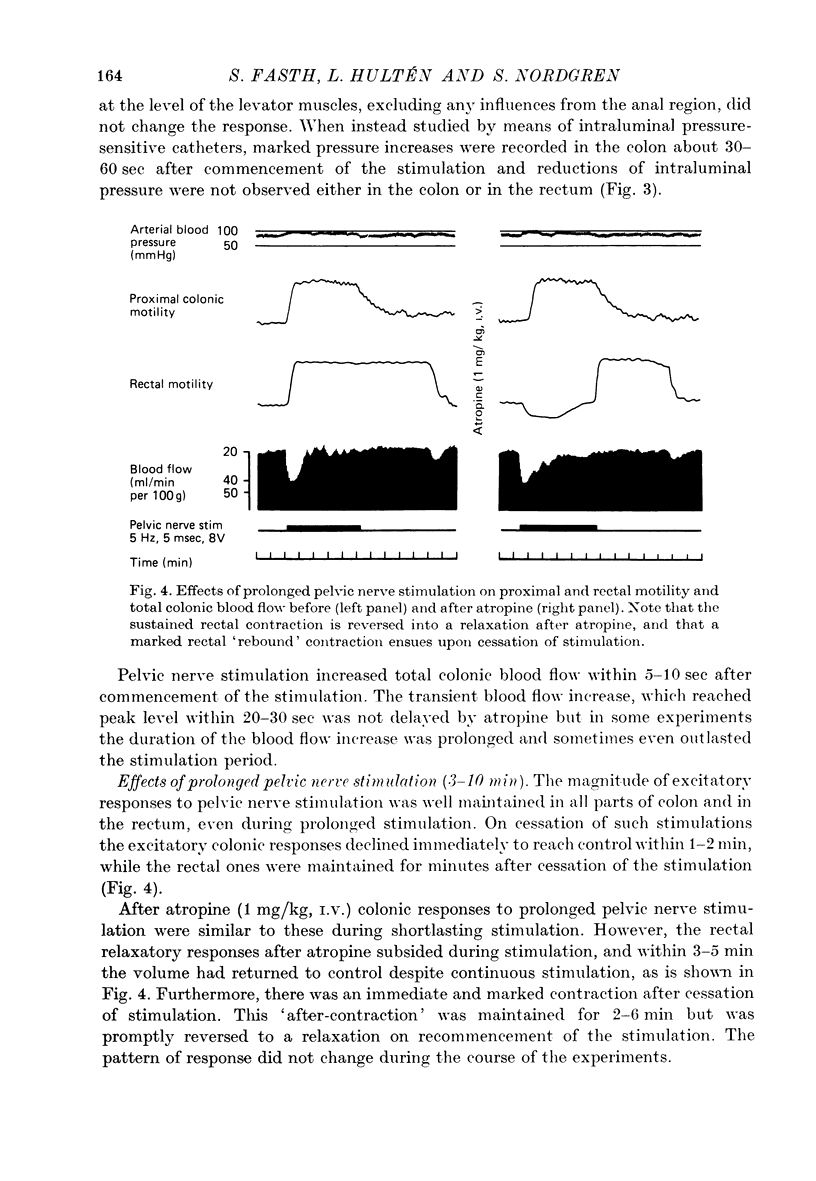
2. Pelvic nerve stimulation induced immediate and sustained colorectal contractions and a simultaneous increase of the over-all colonic blood flow. The excitatory responses declined immediately on cessation of a shortlasting stimulation (< 2 min); after a longlasting one, however, the rectal contraction was maintained for several min.
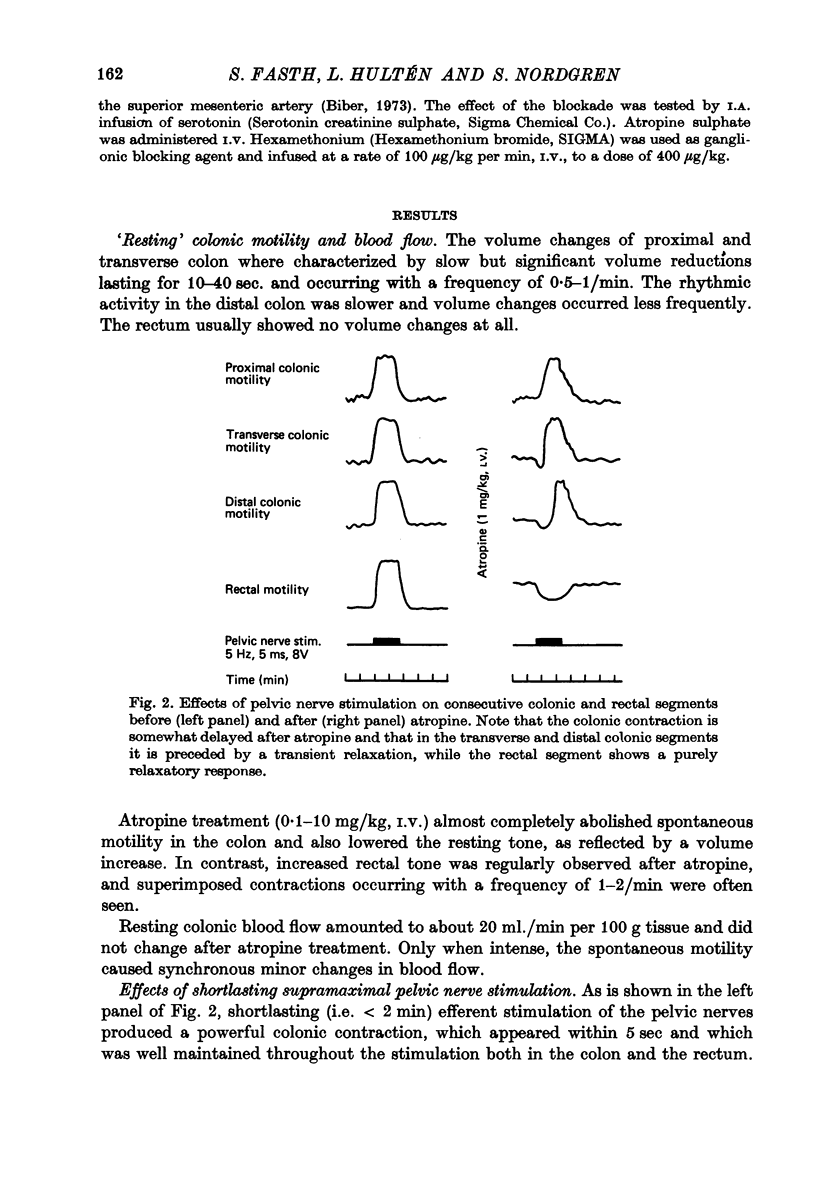
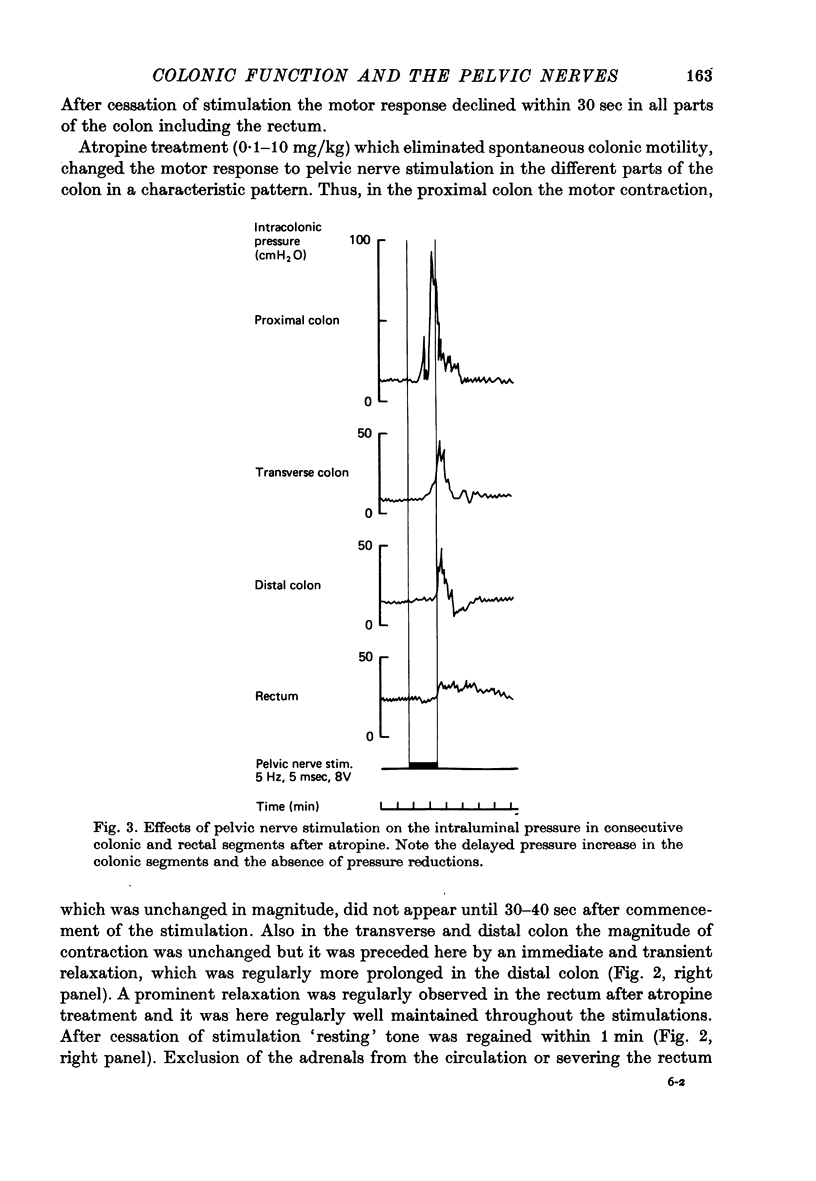
3. The colonic contraction on pelvic nerve stimulation remained unchanged after atropine but was delayed in onset. Moreover, in the transverse and distal colon it was preceded by a relaxation which was most pronounced in the distal part. The vasodilator response was unchanged.
4. After atropine the rectal segment showed a purely relaxatory response. Despite continuous pelvic nerve stimulation the relaxation vanished, however, and rectal volume returned to resting level with 3-5 min. On cessation of such a prolonged stimulation there was a marked rectal `after-contraction'.
5. The excitation thresholds for the efferent nerve fibres eliciting these different responses could not be separated. The motility and the vasodilator responses were not influenced by adrenergic or by serotoninergic blockade.
6. The results indicate that direct preganglionic stimulation of the cat pelvic nerves activates intramural cholinergic excitatory neurones as well as non-cholinergic excitatory neurones and furthermore, non-adrenergic non-cholinergic inhibitory neurones, which together result in most complex colonic and rectal motor responses. From a functional point of view these centrally controlled responses may well be independently controlled by separate preganglionic neurones though they do not differ concerning excitation thresholds.
7. The effects are consistent with a dual function of the distal colon and rectum. Such a dual parasympathetic influence on the large bowel simulates the vagal control of the stomach, where specific vagal relaxatory fibres convey a reflex widening of the corpus-fundus reservoir during food intake.
Full text
PDF

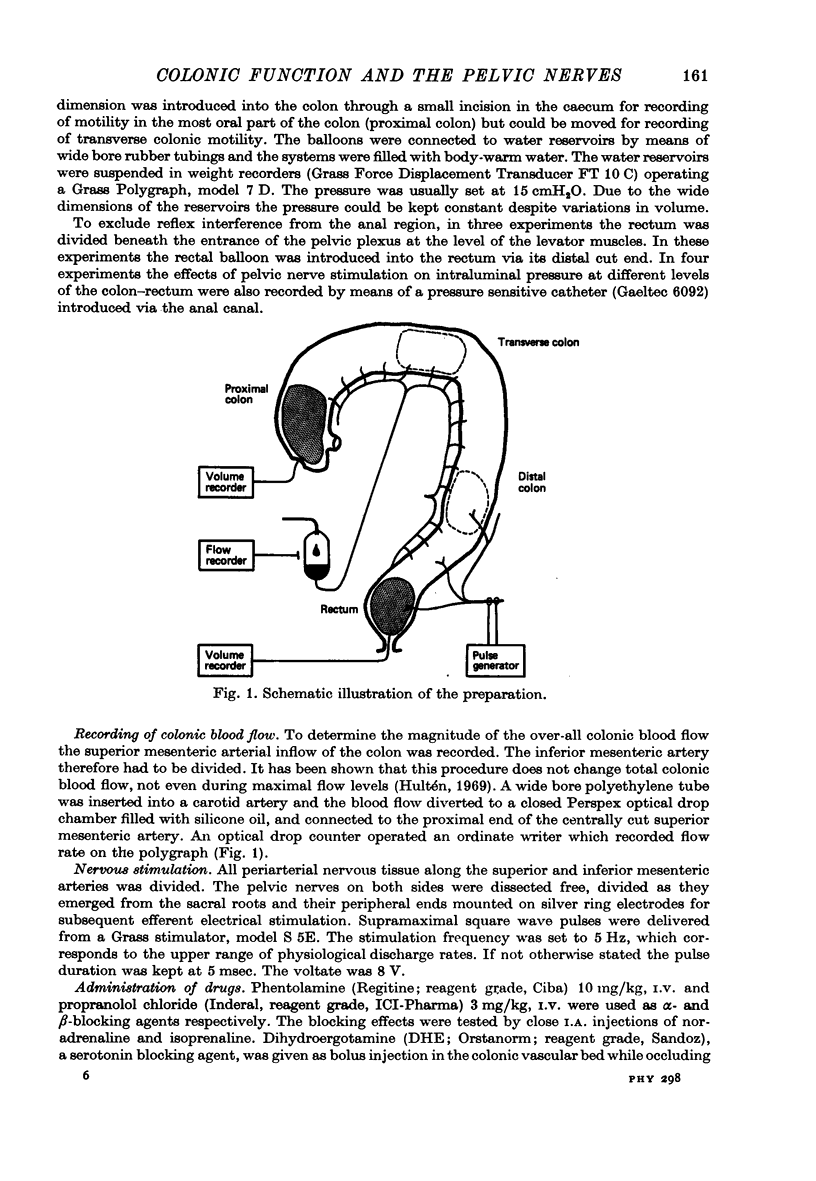





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1–38. [PubMed] [Google Scholar]
- Ambache N., Freeman M. A. Atropine-resistant longitudinal muscle spasms due to excitation of non-cholinergic neurones in Auerbach's plexus. J Physiol. 1968 Dec;199(3):705–727. doi: 10.1113/jphysiol.1968.sp008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. An inhibitory action of histamine on the guinea-pig ileum. Br J Pharmacol. 1970 Jan;38(1):229–240. doi: 10.1111/j.1476-5381.1970.tb10352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INHIBITION OF THE SMOOTH MUSCLE ON THE TAENIA COLI. Nature. 1963 Nov 9;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The movements and the innervation of the large intestine. J Physiol. 1900 Dec 31;26(1-2):107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Stockley H. L. The intrinsic innervation of the human alimentary tract and its relation to function. Gut. 1975 Jun;16(6):443–453. doi: 10.1136/gut.16.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber B. Vasodilator mechanisms in the small intestine. An experimental study in the cat. Acta Physiol Scand Suppl. 1973;401:1–31. [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Campbell G. Nerve-mediated excitation of the taenia of the guinea-pig caecum. J Physiol. 1966 Jul;185(1):148–159. doi: 10.1113/jphysiol.1966.sp007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema A., Del Tacca M., Frigo G. M., Lecchini S. Presence of a non-adrenergic inhibitory system in the human colon. Gut. 1968 Dec;9(6):633–637. doi: 10.1136/gut.9.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUELGRAFF G., SCHMIDT L., AZOKWU P. UBER DIE ATROPINRESISTENTE NEUROMUSKULAERE UBERTRAGUNG AM PELVICUS-COLON-PRAEPARAT DER KATZE. Arch Int Pharmacodyn Ther. 1964 Jun 1;149:537–551. [PubMed] [Google Scholar]
- FUELGRAFF G., SCHMIDT L. ZUR FRAGE DER HUMORAL VERURSACHTEN, ATROPINRESISTENTEN KONTRAKTION AM PELVICUS-COLONPRAEPARAT VON KATZEN. Med Exp Int J Exp Med. 1963;9:378–384. [PubMed] [Google Scholar]
- Fasth S., Hulten L., Johnson B. J., Nordgren S., Zeitlin I. J. Mobilization of colonic kallikrein following pelvic nerve stimulation in the atropinized cat. J Physiol. 1978 Dec;285:471–478. doi: 10.1113/jphysiol.1978.sp012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth S., Hultén L., Lundgren O., Nordgren S. Vascular responses to mechanical stimulation of the mucosa of the cat colon. Acta Physiol Scand. 1977 Sep;101(1):98–104. doi: 10.1111/j.1748-1716.1977.tb05987.x. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The nervous release and the action of substances which affect intestinal muscle through neither adrenoreceptors nor cholinoreceptors. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):123–133. doi: 10.1098/rstb.1973.0015. [DOI] [PubMed] [Google Scholar]
- Furness J. B. Secondary excitation of intestinal smooth muscle. Br J Pharmacol. 1971 Feb;41(2):213–226. doi: 10.1111/j.1476-5381.1971.tb08023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. The presence of inhibitory nerves in the colon after sympathetic denervation. Eur J Pharmacol. 1969;6(3):349–352. doi: 10.1016/0014-2999(69)90196-4. [DOI] [PubMed] [Google Scholar]
- GARRY R. C., GILLESPIE J. S. The responses of the musculature of the colon of the rabbit to stimulation, in vitro, of the parasympathetic and of the sympathetic outflows. J Physiol. 1955 Jun 28;128(3):557–576. doi: 10.1113/jphysiol.1955.sp005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. R., Howard E. R., Jones W. The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol. 1974 Nov;243(1):153–166. doi: 10.1113/jphysiol.1974.sp010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg M. M., Burns R. H. Atropine-resistant spasm of the dog colon induced by intermittent pelvic nerve stimulation. Life Sci I. 1971 May 15;10(10):591–600. doi: 10.1016/0024-3205(71)90045-2. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On the Innervation of the Pelvic and Adjoining Viscera: Part I. The Lower Portion of the Intestine. J Physiol. 1895 May 20;18(1-2):67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga A., Taneike T. Dissimilarity between the responses to adenosine triphosphate or its related compounds and non-adrenergic inhibitory nerve stimulation in the longitudinal smooth muscle of pig stomach. Br J Pharmacol. 1977 Jun;60(2):221–231. doi: 10.1111/j.1476-5381.1977.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikimaru A., Fukushi Y., Suzuki T. Evidence for the presence of non-adrenergic inhibitory nerves in the human taenia coli. Tohoku J Exp Med. 1971 Jun;104(2):199–200. doi: 10.1620/tjem.104.199. [DOI] [PubMed] [Google Scholar]
- Rostad H. Colonic motility in the cat. II. Extrinsic nervous control. Acta Physiol Scand. 1973 Sep;89(1):91–103. doi: 10.1111/j.1748-1716.1973.tb05500.x. [DOI] [PubMed] [Google Scholar]


