Abstract
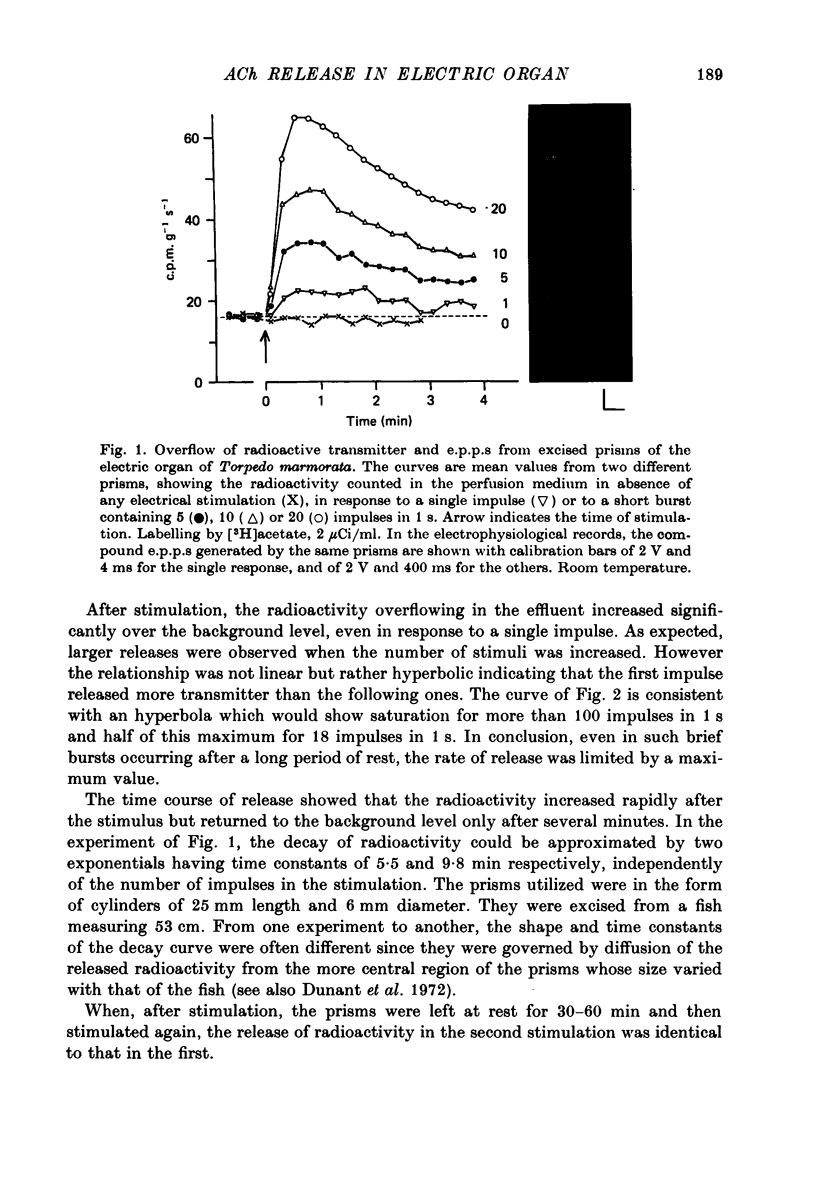
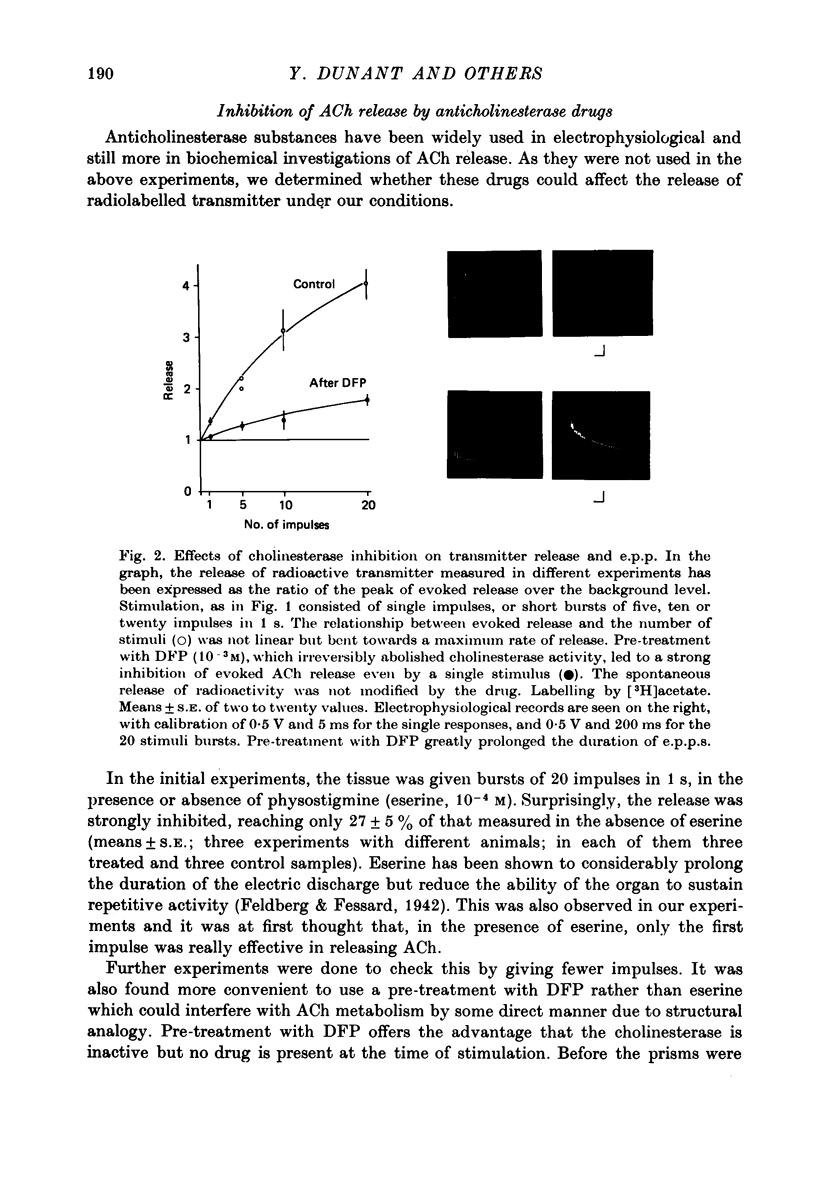
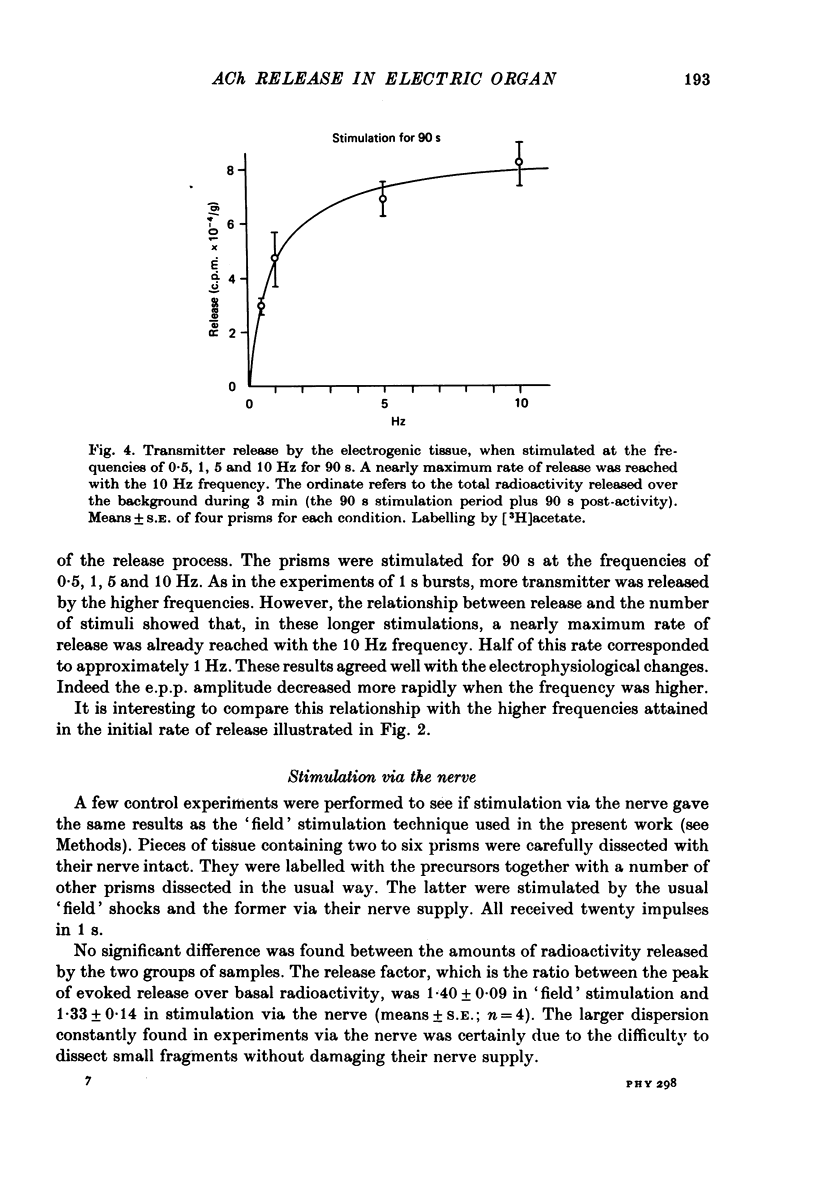
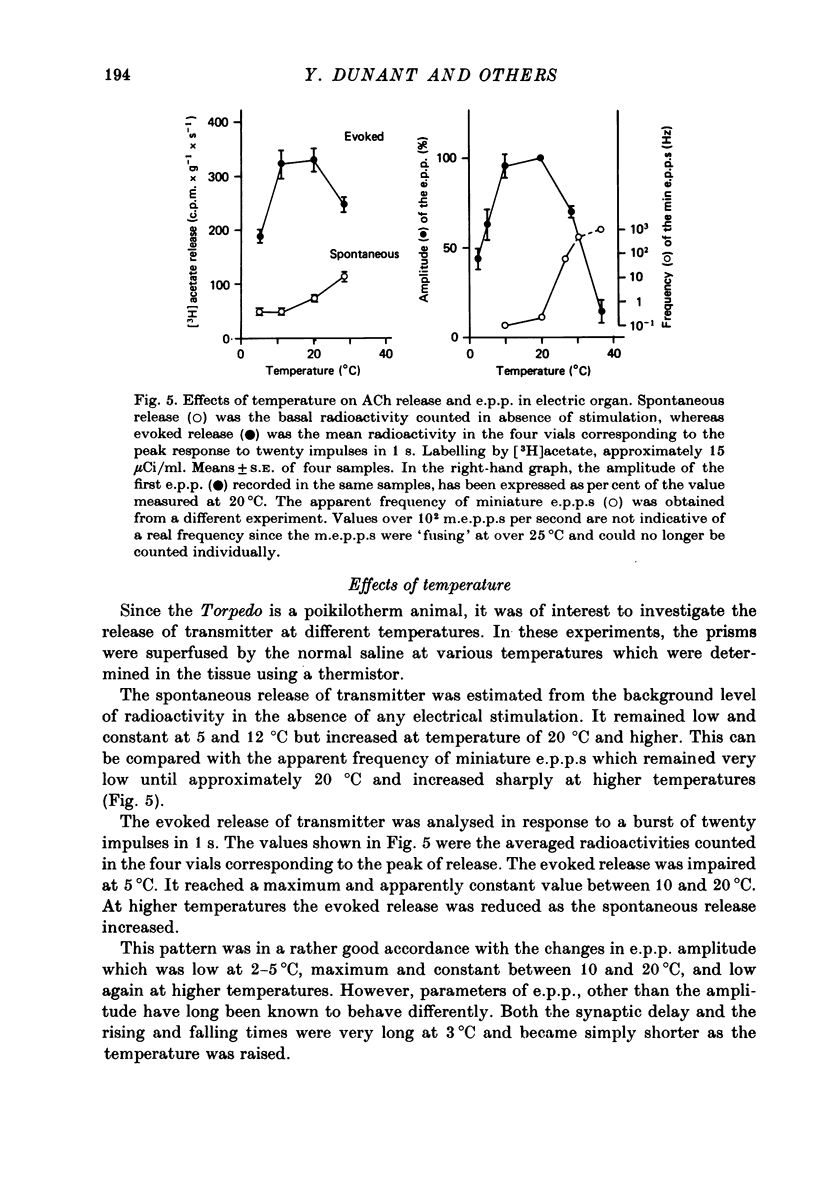
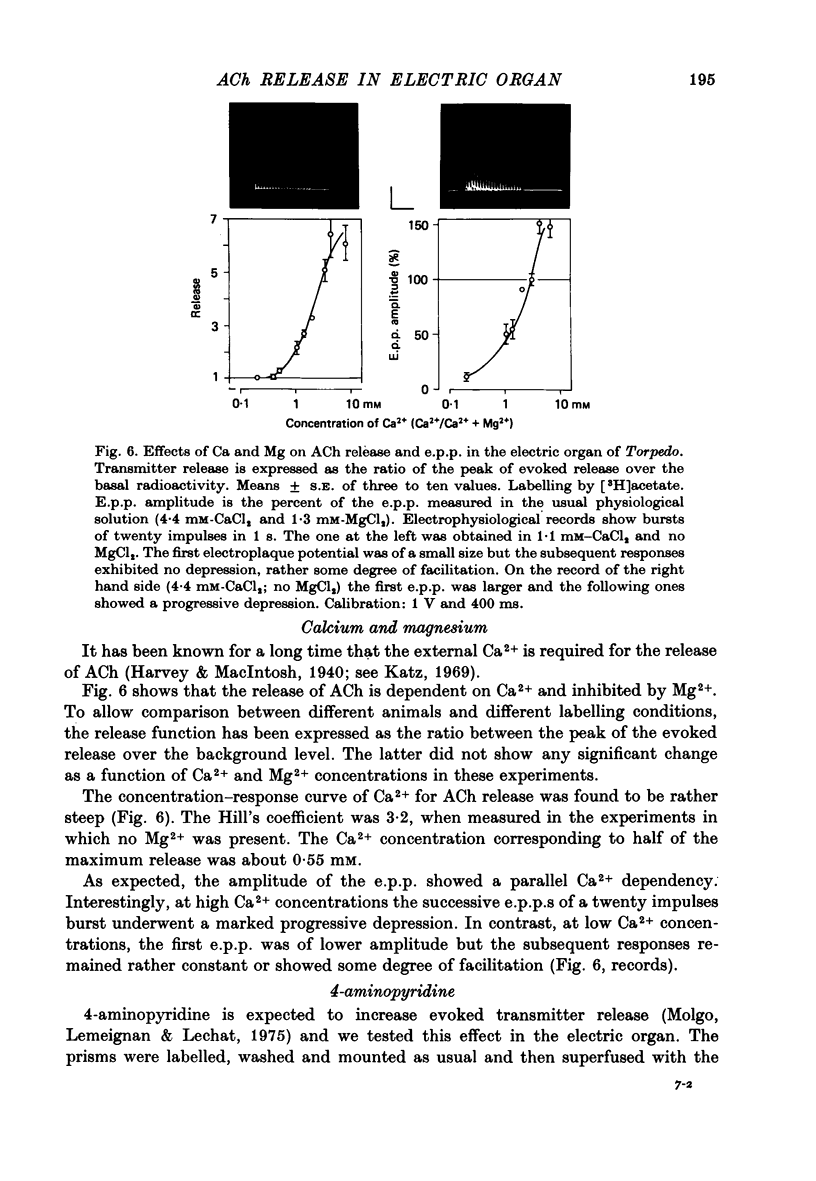
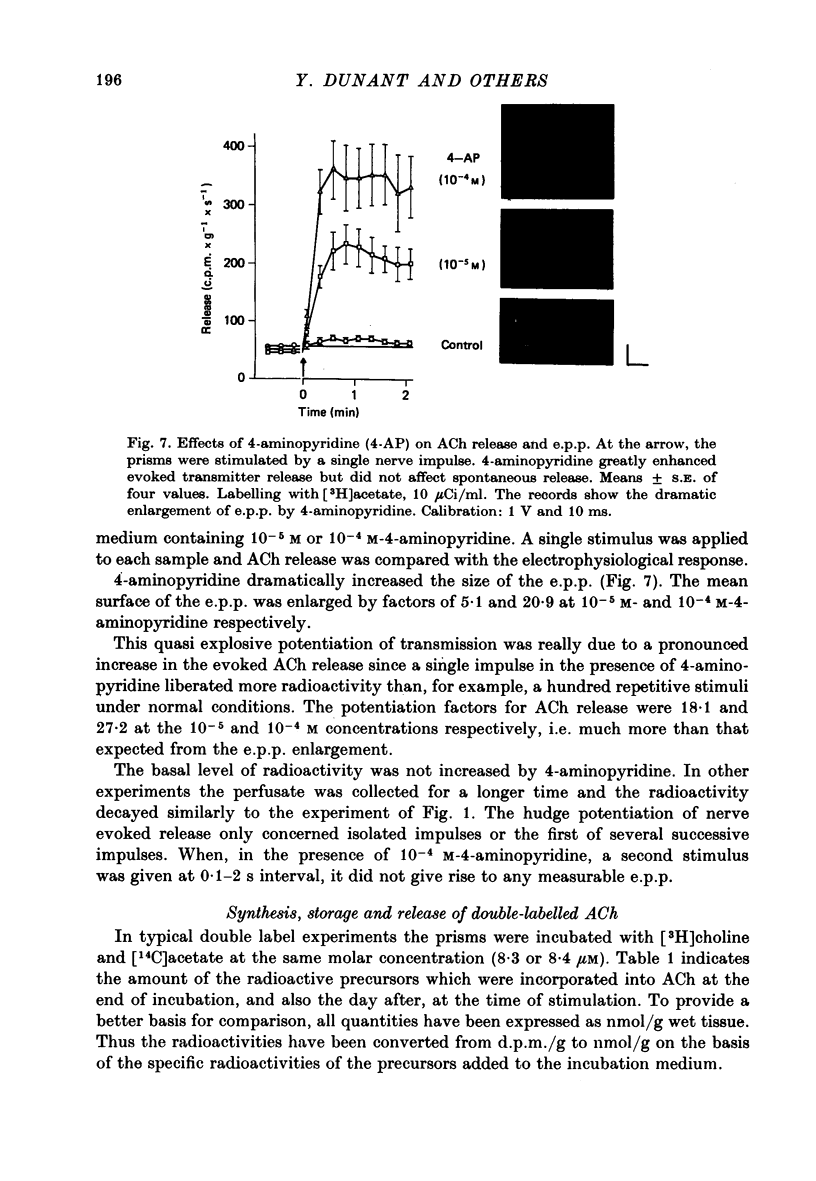
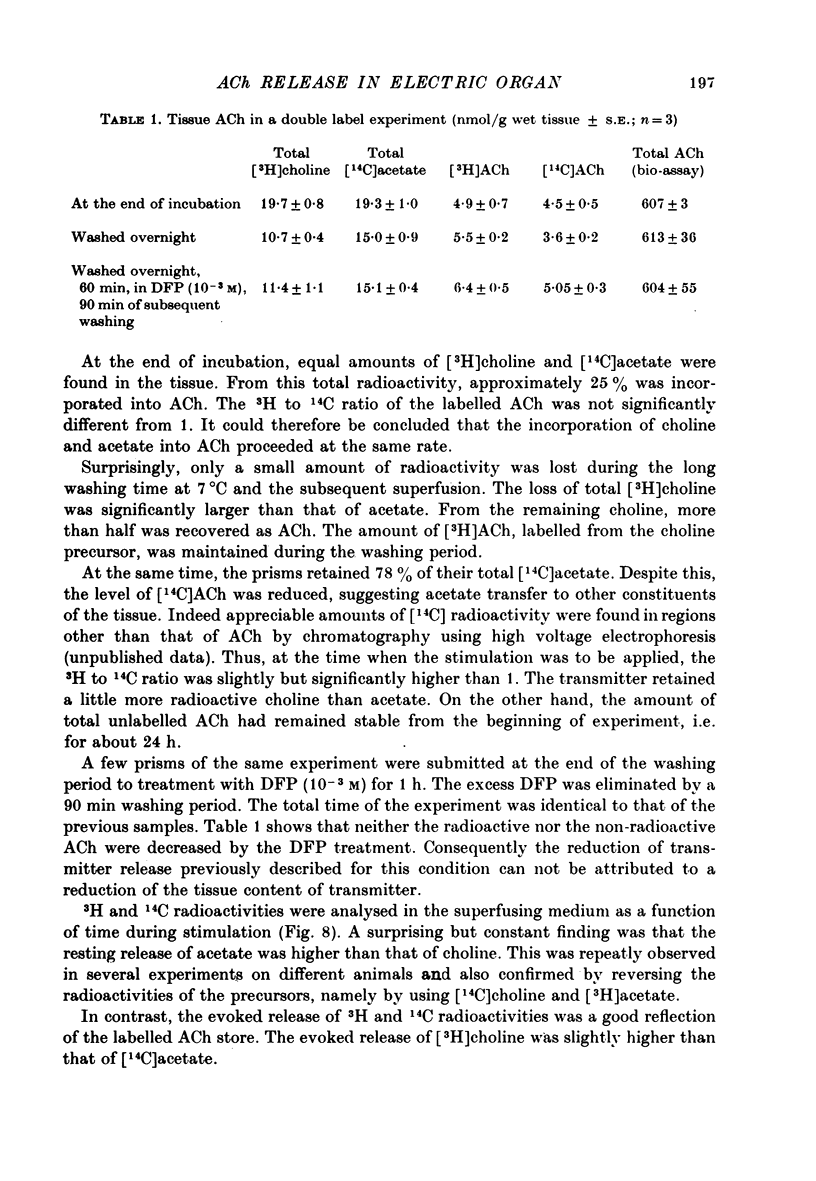
1. The acetylcholine (ACh) store in the Torpedo electric organ was partially labelled with choline and acetate at the same molar concentration but with different isotopes. Under these conditions the two precursors were incorporated into ACh in a ratio 1 to 1. 2. After a single electrical stimulus, or a brief burst of stimuli, the compound electroplaque potential (e.p.p.) was recorded and the radioactive choline and/or acetate counted in the perfusion fluid, providing a sensitive assay for ACh release in the absence of anticholinesterase drugs. 3. The so-called depression of transmission was found to be due to progressive impairment of ACh release in the successive impulses evoked by repeated stimuli. 4. In a pair of impulses separated by 50 ms interval, less ACh was released by the second than by the first impulse; this explained why the size of the second e.p.p. was depressed, using a direct measurement of ACh. 5. In repetitive stimulations of longer duration, the maximum rate of release declined as the activity was prolonged. Thus the tissue progressively lost its ability to ensure release at high frequencies. 6. An unexpected finding was that anticholinesterases like eserine or pre-treatment with fluostigmine (DFP) greatly reduced ACh release even by a single impulse. 7. Evoked ACh release and e.p.p. amplitude were both maximum between 10 and 20 degrees C. At higher temperatures, the evoked release decreased as the spontaneous release increased. 8. Changes in external Ca2+ and Mg2+ produced similar changes in the e.p.p. and evoked ACh release. The dose--response curve for Ca dependency of ACh release was very steep with a Hill's coefficient of 3.2. 9. With a single stimulus in the presence of 4-aminopyridine, there was a dramatic enlargement of the e.p.p. and a still larger potentiation of the evoked ACh release. 10. It has been possible with this approach to avoid the inconveniences often encountered in simliar studies, i.e. repetitive stimulation, low Ca solutions and cholinesterase inhibition. This permitted a good correlation between electrophysiological and biochemical estimates of transmitter release even by a single nerve impulse.
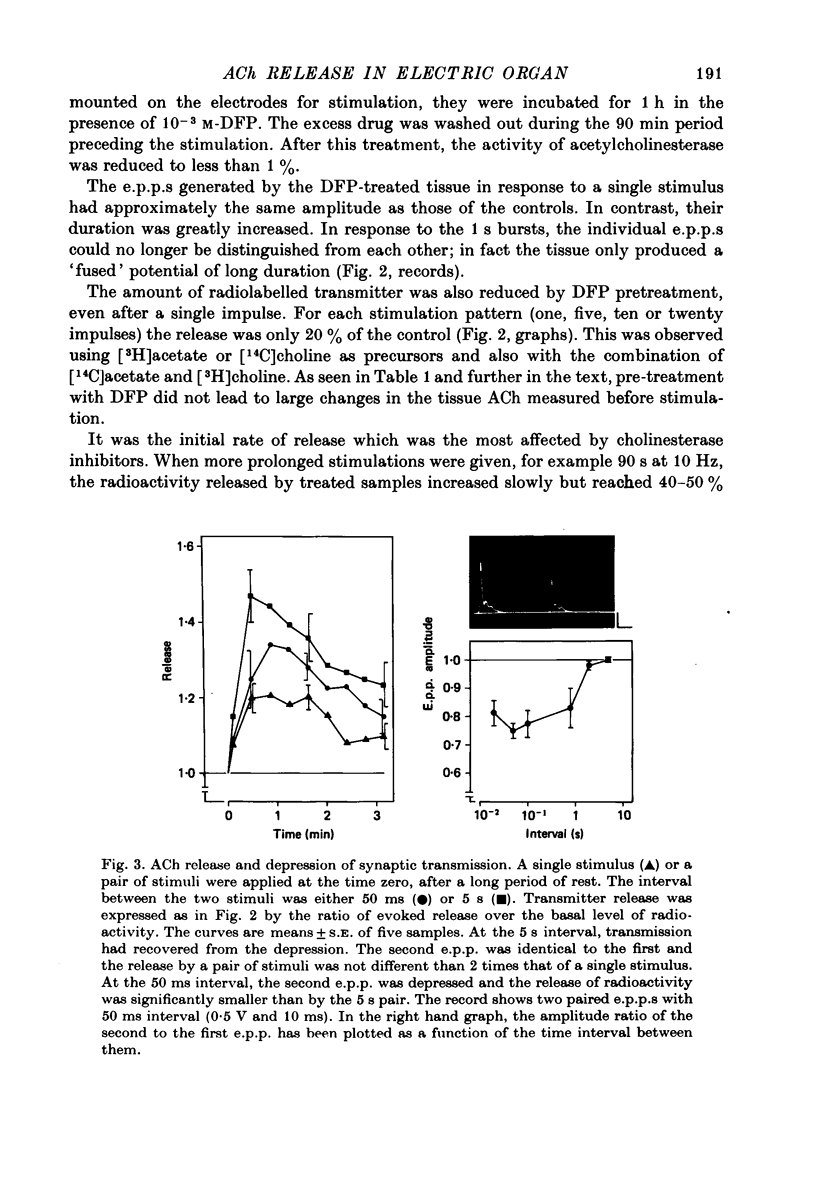
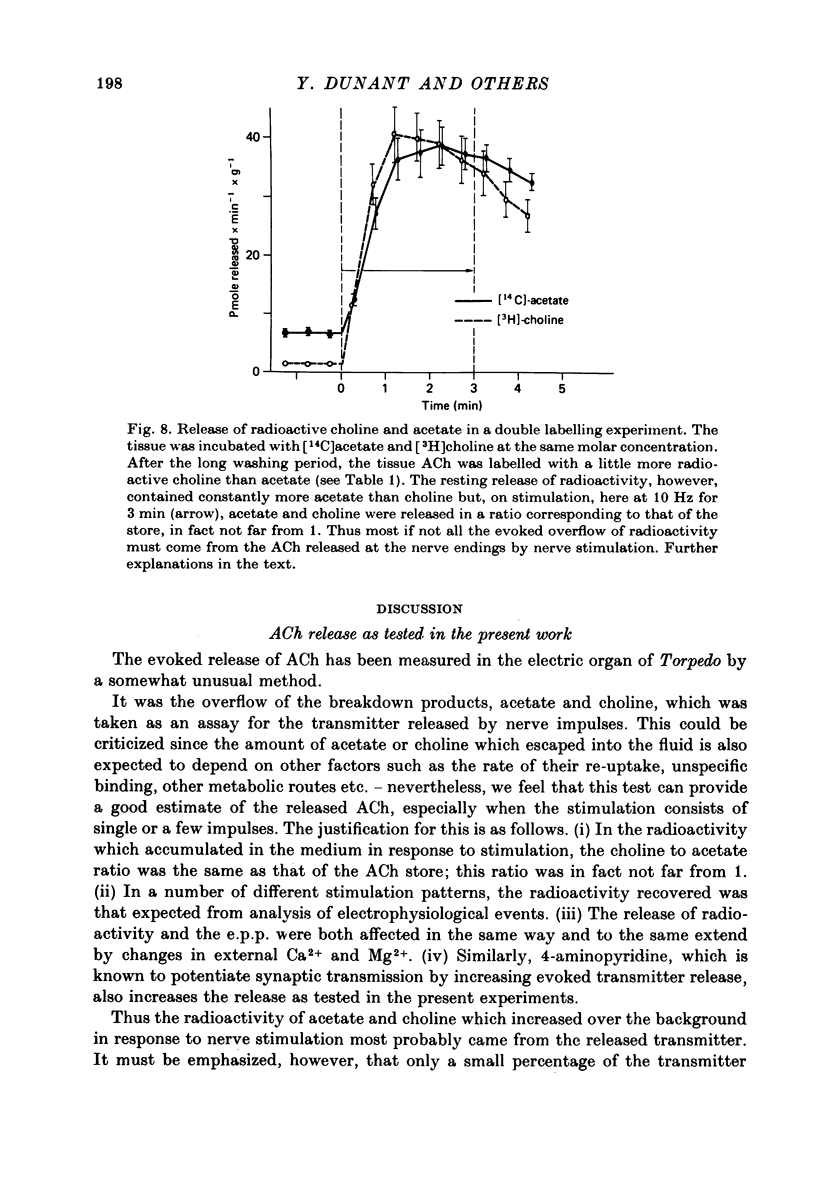
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babel-Guérin E. Metabolisme du calcium et liberation de l'acetylcholine dans l'organe electrique de la Torpille. J Neurochem. 1974 Sep;23(3):525–532. doi: 10.1111/j.1471-4159.1974.tb06055.x. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Feldberg W. The acetyloholine metabolism of a sympathetic ganglion. J Physiol. 1936 Dec 11;88(3):265–283. doi: 10.1113/jphysiol.1936.sp003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y. Acetylcholine metabolism and release at the nerve-electroplaque junction. Brain Res. 1973 Nov 23;62(2):543–549. doi: 10.1016/0006-8993(73)90720-8. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Evolution de la décharge de l'organe électrique de la Torpille et variations simultanées de l'acétylcholine au cours de la stimulation. J Neurochem. 1974 Oct;23(4):635–643. doi: 10.1111/j.1471-4159.1974.tb04386.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Les compartiments d'acetylcholine de l'organe electrique de la torpille et leurs modifications par la stimulation. J Neurochem. 1972 Aug;19(8):1987–2002. doi: 10.1111/j.1471-4159.1972.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Hirt L. A rapid radiochemical assay for acetylcholine. J Neurochem. 1976 Mar;26(3):657–659. doi: 10.1111/j.1471-4159.1976.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Israël M., Lesbats B., Manaranche R. Loss of vesicular acetycholine in the Torpedo electric organ on discharge against high external resistance. J Neurochem. 1976 Oct;27(4):975–977. doi: 10.1111/j.1471-4159.1976.tb05166.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Israël M., Lesbats B., Manaranche R. Oscillation of acetylcholine during nerve activity in the Torpedo electric organ. Brain Res. 1977 Apr 8;125(1):123–140. doi: 10.1016/0006-8993(77)90364-x. [DOI] [PubMed] [Google Scholar]
- Eder L., Hirt L., Dunant Y. Possible involvement of thiamine in acetylcholine release. Nature. 1976 Nov 11;264(5582):186–188. doi: 10.1038/264186a0. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Fessard A. The cholinergic nature of the nerves to the electric organ of the Torpedo (Torpedo marmorata). J Physiol. 1942 Aug 18;101(2):200–216. doi: 10.1113/jphysiol.1942.sp003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Lesbats B., Manaranche R., Marsal J., Mastour-Frachon P., Meunier F. M. Related changes in amounts of ACh and ATP in resting and active Torpedo nerve electroplaque synapses. J Neurochem. 1977 Jun;28(6):1259–1267. doi: 10.1111/j.1471-4159.1977.tb12319.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Tucek S. Utilization of acetate and pyruvate for the synthesis of 'total', 'bound' and 'free' acetylcholine in the electric organ of Torpedo. J Neurochem. 1974 Apr;22(4):487–491. doi: 10.1111/j.1471-4159.1974.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Meunier F., Israël M., Lesbats B. Release of ATP from stimulated nerve electroplaque junctions. Nature. 1975 Oct 2;257(5525):407–408. doi: 10.1038/257407a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978 Apr 13;272(5654):641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Modifications de la libération du transmetteur à la jonction neuromusculaire de grenouille sous l'action de l'amino-4 pyridine. C R Acad Sci Hebd Seances Acad Sci D. 1975 Nov 24;281(21):1637–1639. [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- PERRY W. L. M. Acetylcholine release in the cat's superior cervical ganglion. J Physiol. 1953 Mar;119(4):439–454. doi: 10.1113/jphysiol.1953.sp004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Consolo S., Peri G., Prigioni I., Ladinsky H., Perri V. Storage and release of acetylcholine in the isolated superior cervical ganglion of the rat. Brain Res. 1978 Aug 11;151(3):443–456. doi: 10.1016/0006-8993(78)91078-8. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Somogyi G. T. Depression of acetylcholine release from cerebral cortical slices by cholinesterase inhibition and by oxotremorine. Nat New Biol. 1973 Jan 24;241(108):121–122. doi: 10.1038/newbio241121a0. [DOI] [PubMed] [Google Scholar]


