Abstract
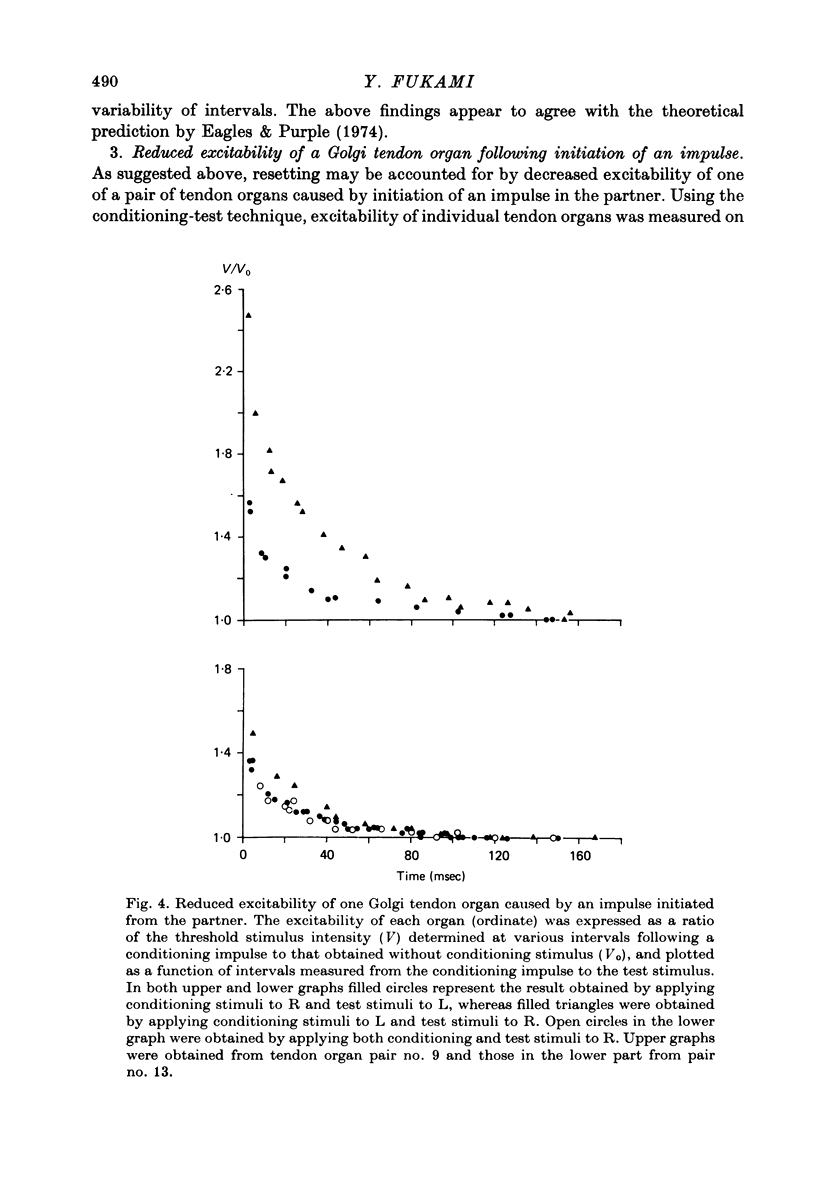
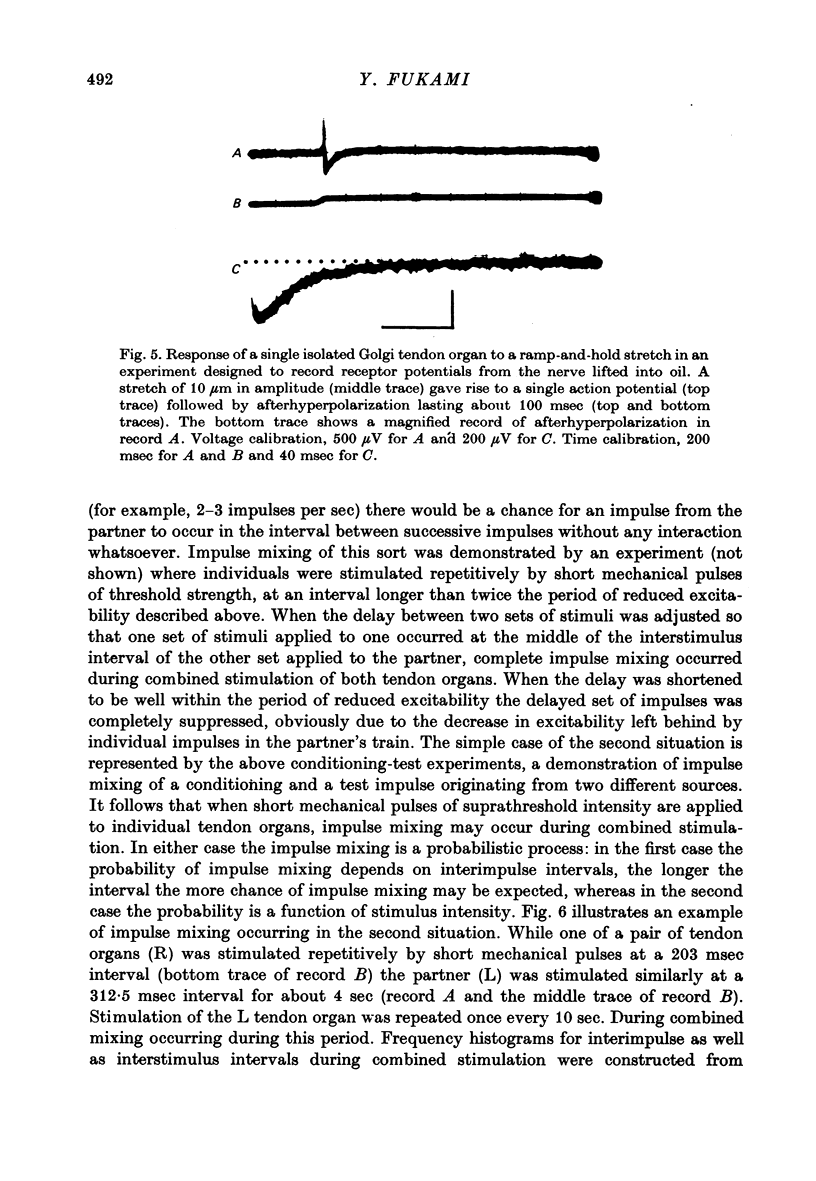
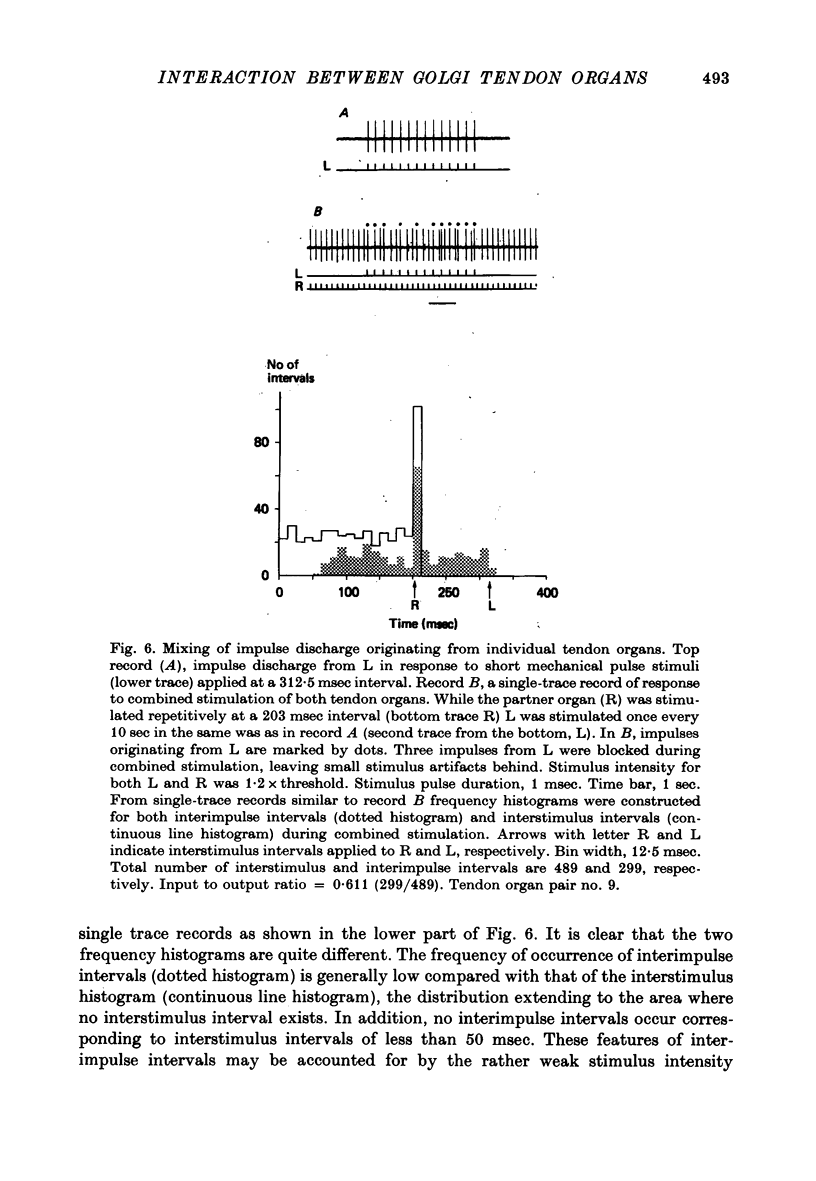
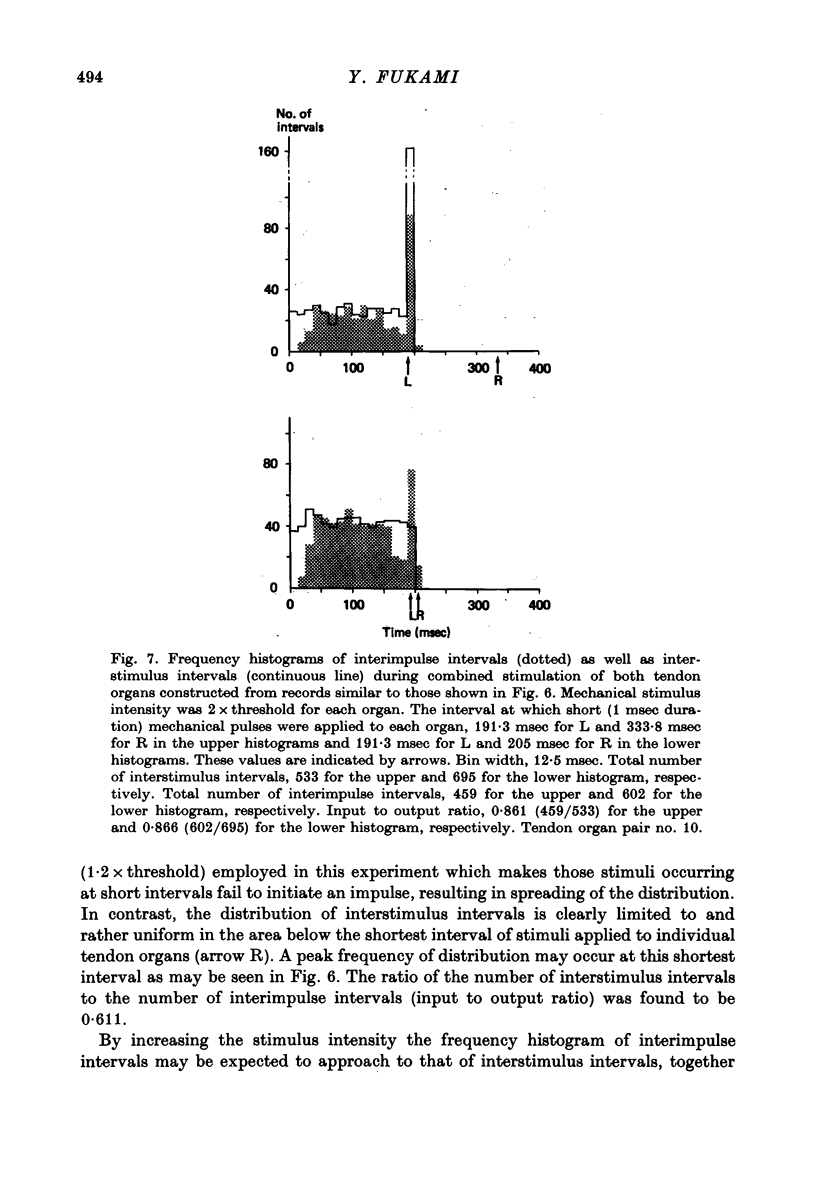
1. Both physiological and morphological studies revealed that cat tail muscles contain at least one pair of Golgi tendon organs innervated by branches of a single axon. 2. Fifteen pairs of such organs were subjected to physiological studies. It was found that, depending on the experimental conditions, two modes of interaction, 'resetting' and 'impulse mixing' may occur between impulse activities originating from individual tendon organs. 3. When a single action potential was elicited from one of a pair of Golgi tendon organs during an interimpulse interval of a train of impulse discharge originating from the partner organ, the subsequent impulses in the train were delayed ('resetting'). Similarly, if both organs were stimulated individually by a mechanical pulse to elicit a train of discharge, then during stimulation of both, only the response of one responding with higher frequency discharge was seen in the parent axon, the impulse activity of the partner organ being completely suppressed during this period. 4. Using the conditioning-test technique it was demonstrated that the initiation of an action potential in one of a pair of Golgi tendon organs caused a significant decrease in excitability of the partner organ to mechanical stimulation. 5. Mixing of impulse discharges originating from individual Golgi tendon organs was shown to occur during stimulation of both by suprathreshold short mechanical pulses. 6. The functional implication of the above results has been discussed.
Full text
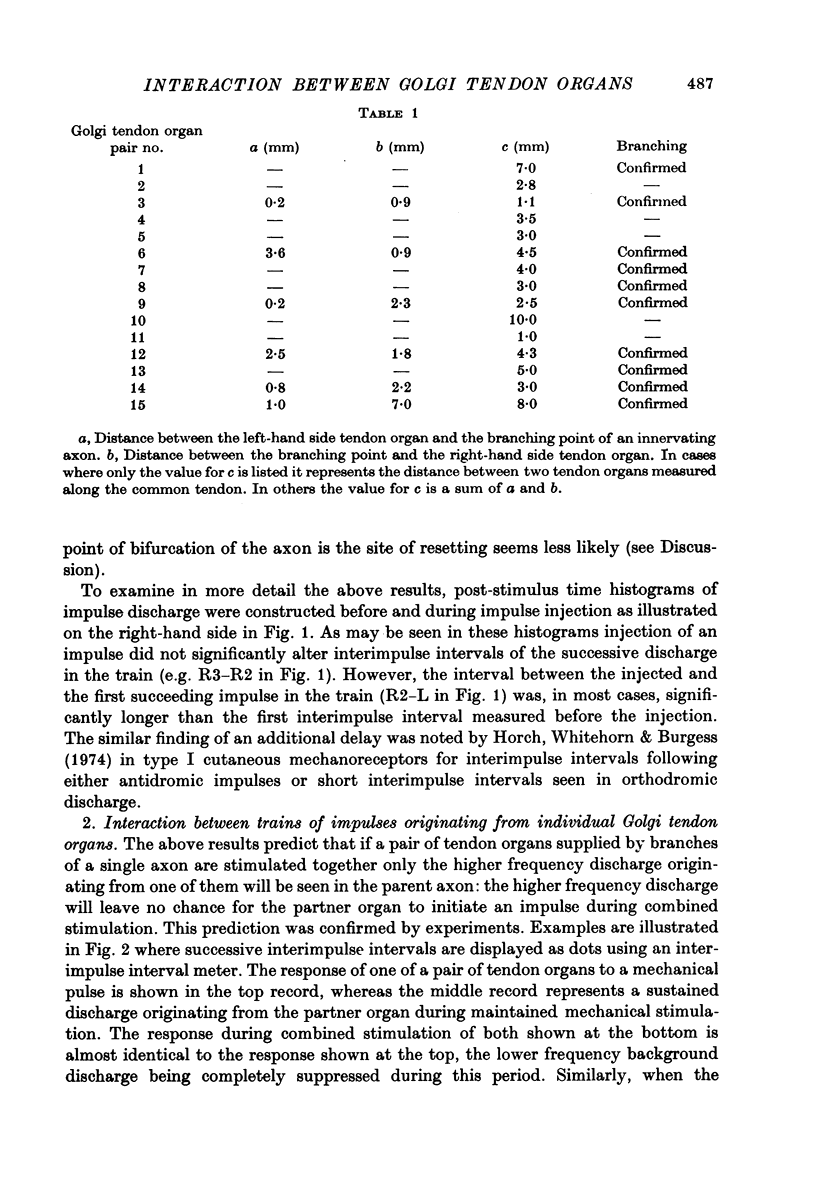
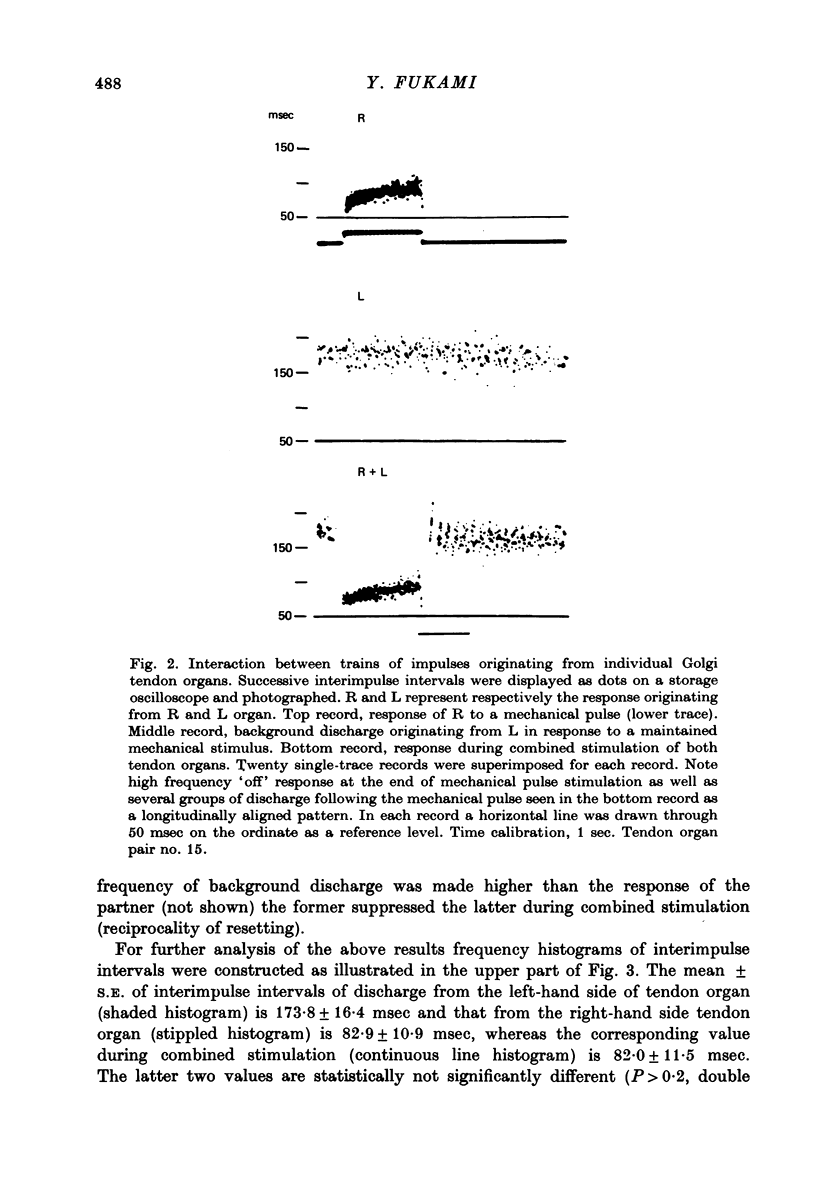
PDF


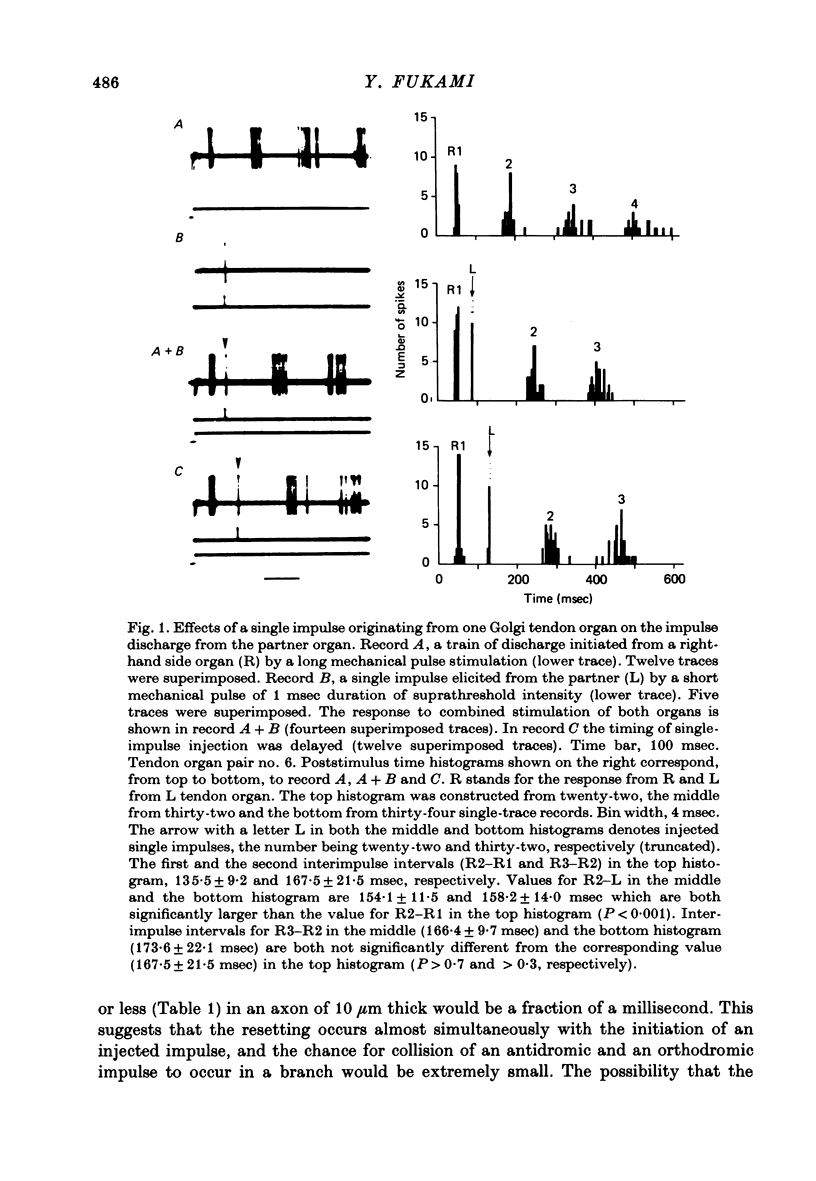






Selected References
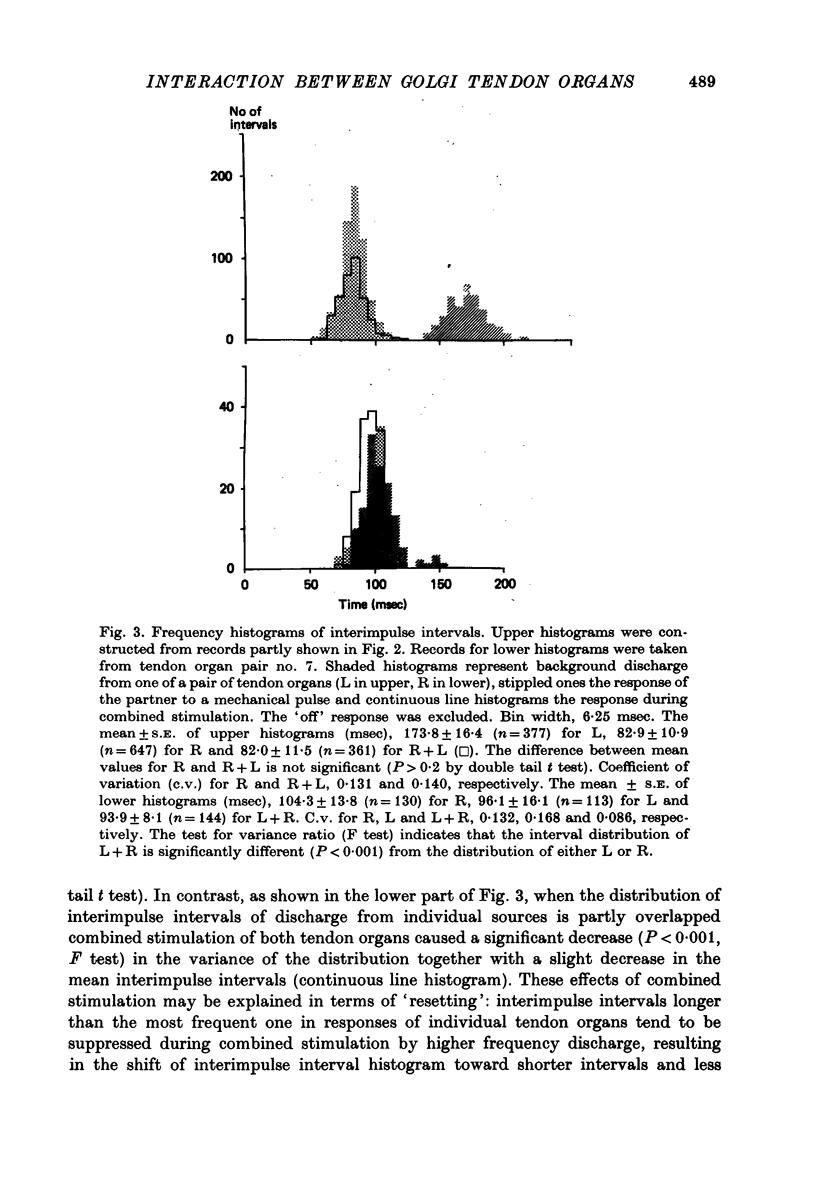
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926 Apr 23;61(2):151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stuart D. G. The response of Golgi tendon organs to single motor unit contractions. J Physiol. 1977 Oct;271(2):337–349. doi: 10.1113/jphysiol.1977.sp012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokensha G., Westbury D. R. Adaptation of the discharge of frog muscle spindles following a stretch. J Physiol. 1974 Oct;242(2):383–403. doi: 10.1113/jphysiol.1974.sp010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclaux R., Kenshalo D. R. Cutaneous receptive fields of primate cold fibers. Brain Res. 1973 Jun 15;55(2):437–442. doi: 10.1016/0006-8993(73)90309-0. [DOI] [PubMed] [Google Scholar]
- Eagles J. P., Purple R. L. Afferent fibers with multiple encoding sites. Brain Res. 1974 Sep 6;77(2):187–193. doi: 10.1016/0006-8993(74)90783-5. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Hulliger M., Matthews P. B., Petit J. Factors affecting modulation in post-stimulus histograms on static fusimotor stimulation. Brain Res. 1977 Sep 23;134(1):180–184. doi: 10.1016/0006-8993(77)90938-6. [DOI] [PubMed] [Google Scholar]
- Floyd K., Morrison J. F. Proceedings: Interactions between afferent impulses within a peripheral receptive field. J Physiol. 1974 Apr;238(1):62P–63P. [PubMed] [Google Scholar]
- Fukami Y., Wilkinson R. S. Responses of isolated Golgi tendon organs of the cat. J Physiol. 1977 Mar;265(3):673–689. doi: 10.1113/jphysiol.1977.sp011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Stauffer E. K., Nemeth W. C., Stuart D. G. The cat step cycle; responses of muscle spindles and tendon organs to passive stretch within the locomotor range. Brain Res. 1973 Sep 28;60(1):35–54. doi: 10.1016/0006-8993(73)90849-4. [DOI] [PubMed] [Google Scholar]
- Gregory J. E., Harvey R. J., Proske U. A 'late supernormal period' in the recovery of excitability following an action potential in muscle spindle and tendon organ receptors. J Physiol. 1977 Oct;271(2):449–472. doi: 10.1113/jphysiol.1977.sp012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch K. W., Whitehorn D., Burgess P. R. Impulse generation in type I cutaneous mechanoreceptors. J Neurophysiol. 1974 Mar;37(2):267–281. doi: 10.1152/jn.1974.37.2.267. [DOI] [PubMed] [Google Scholar]
- Houk J. A viscoelastic interaction which produces one component of adaptation in responses of Golgi tendon organs. J Neurophysiol. 1967 Nov;30(6):1482–1493. doi: 10.1152/jn.1967.30.6.1482. [DOI] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Houk J., Simon W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol. 1967 Nov;30(6):1466–1481. doi: 10.1152/jn.1967.30.6.1466. [DOI] [PubMed] [Google Scholar]
- Hunt C. C., Ottoson D. Impulse activity and receptor potential of primary and secondary endings of isolated mammalian muscle spindles. J Physiol. 1975 Oct;252(1):259–281. doi: 10.1113/jphysiol.1975.sp011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Petit J. Frequency of tendon organ discharges elicited by the contraction of motor units in cat leg muscles. J Physiol. 1976 Oct;261(3):633–645. doi: 10.1113/jphysiol.1976.sp011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Petit J. Heterogeneity of motor units activating single Golgi tendon organs in cat leg muscles. Exp Brain Res. 1976 Mar 15;24(5):485–493. doi: 10.1007/BF00234965. [DOI] [PubMed] [Google Scholar]
- KATZ B. Action potentials from a sensory nerve ending. J Physiol. 1950 Oct 16;111(3-4):248–260. doi: 10.1113/jphysiol.1950.sp004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom U., Tapper D. N. Terminal properties of a vibro-tactile sensor. Exp Neurol. 1967 Jan;17(1):1–15. doi: 10.1016/0014-4886(67)90117-3. [DOI] [PubMed] [Google Scholar]
- Lindblom Y., Tapper D. N. Integration of impulse activity in a peripheral sensory unit. Exp Neurol. 1966 May;15(1):63–69. doi: 10.1016/0014-4886(66)90034-3. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. The response of a muscle spindle during active contraction of a muscle. J Physiol. 1931 Jun 26;72(2):153–174. doi: 10.1113/jphysiol.1931.sp002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. Responses of intradental nerves to electrical and thermal stimulation of teeth in dogs. J Physiol. 1977 Jan;264(3):641–664. doi: 10.1113/jphysiol.1977.sp011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I. J., Jr Peripheral interactions among single papilla inputs to gustatory nerve fibers. J Gen Physiol. 1971 Jan;57(1):1–25. doi: 10.1085/jgp.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Gregory J. E. Multiple sites of impulse initiation in a tendon organ. Exp Neurol. 1976 Feb;50(2):515–520. doi: 10.1016/0014-4886(76)90023-6. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stephens J. A., Stuart D. G. The tendon organs of cat medial gastrocnemius: significance of motor unit type and size for the activation of Ib afferents. J Physiol. 1975 Sep;250(3):491–512. doi: 10.1113/jphysiol.1975.sp011067. [DOI] [PMC free article] [PubMed] [Google Scholar]


