Abstract
1. The cortical location and the retinotopic arrangement of the second (V2) and third (V3) visual areas in the cat have been investigated with single and multiple unit recordings in anaesthetized and immobilized animals.
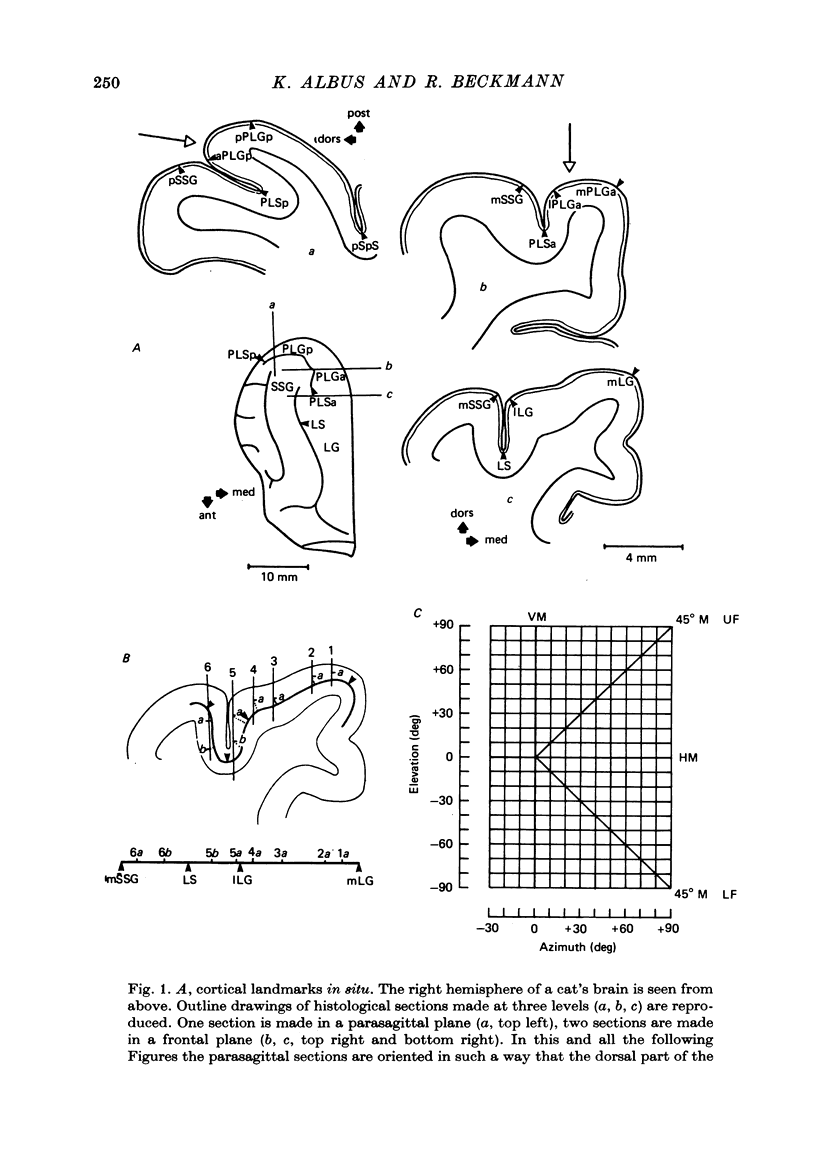
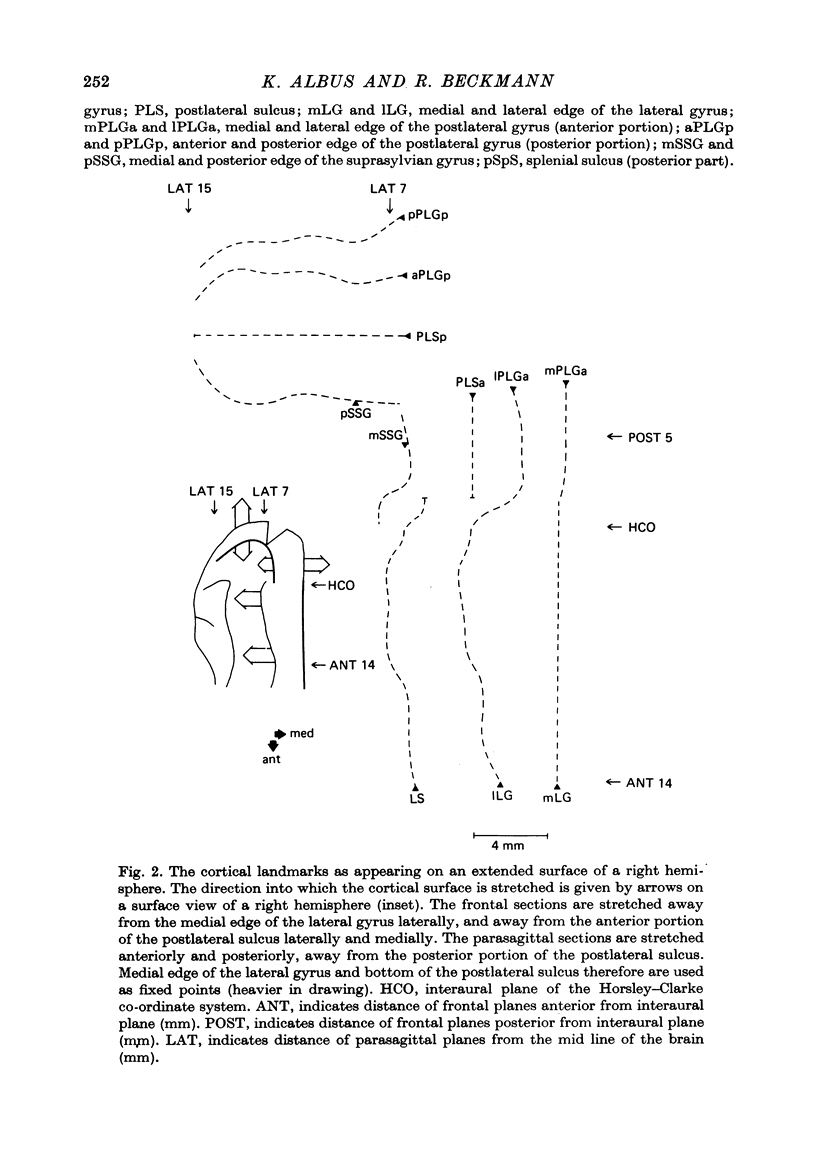
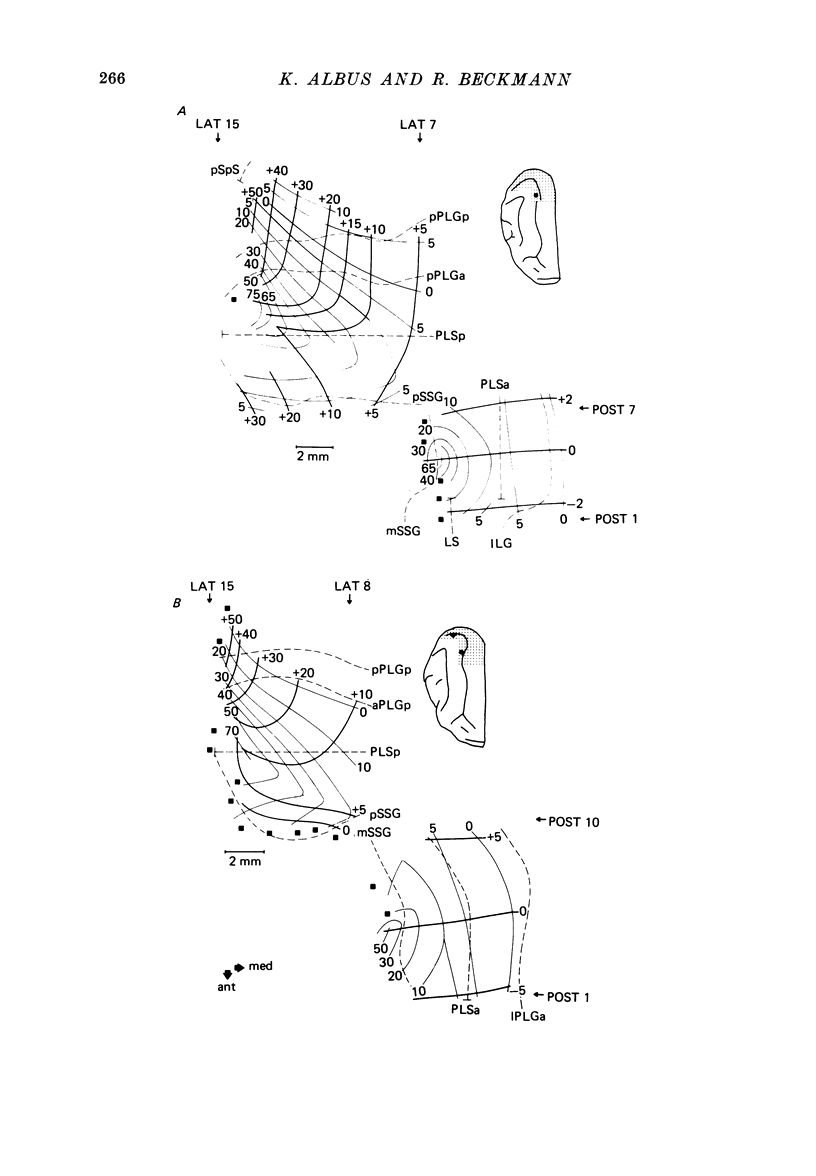
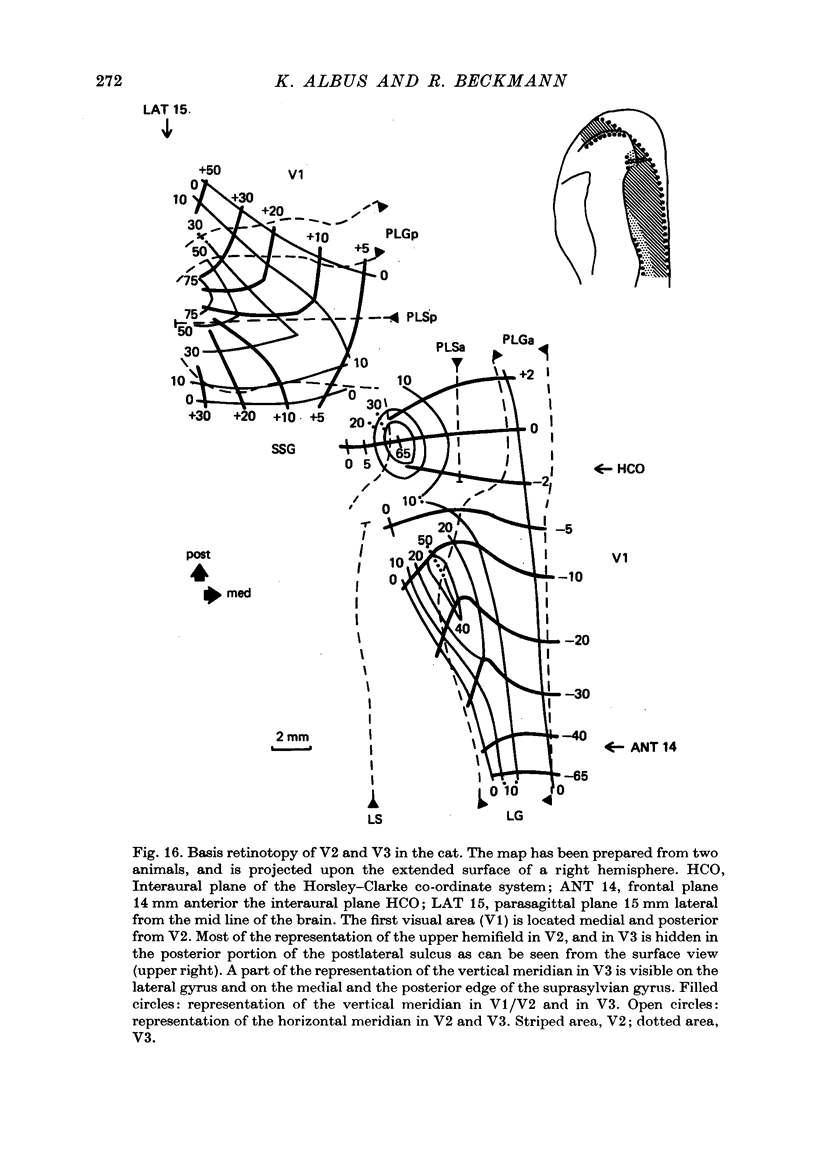
2. V2 and V3 are arranged side by side anterior and medial to V1 and occupy the lateral gyrus and the postlateral sulcus. In addition, V2 spreads to postlateral parts of the lateral sulcus and, occasionally, to the posterior suprasylvian gyrus. The contralateral lower hemifield is represented on the lateral gyrus, the area centralis and the horizontal meridian are found in most animals in the anterior part of the postlateral sulcus, and the representation of the upper hemifield occupies the posterior part of the postlateral sulcus.
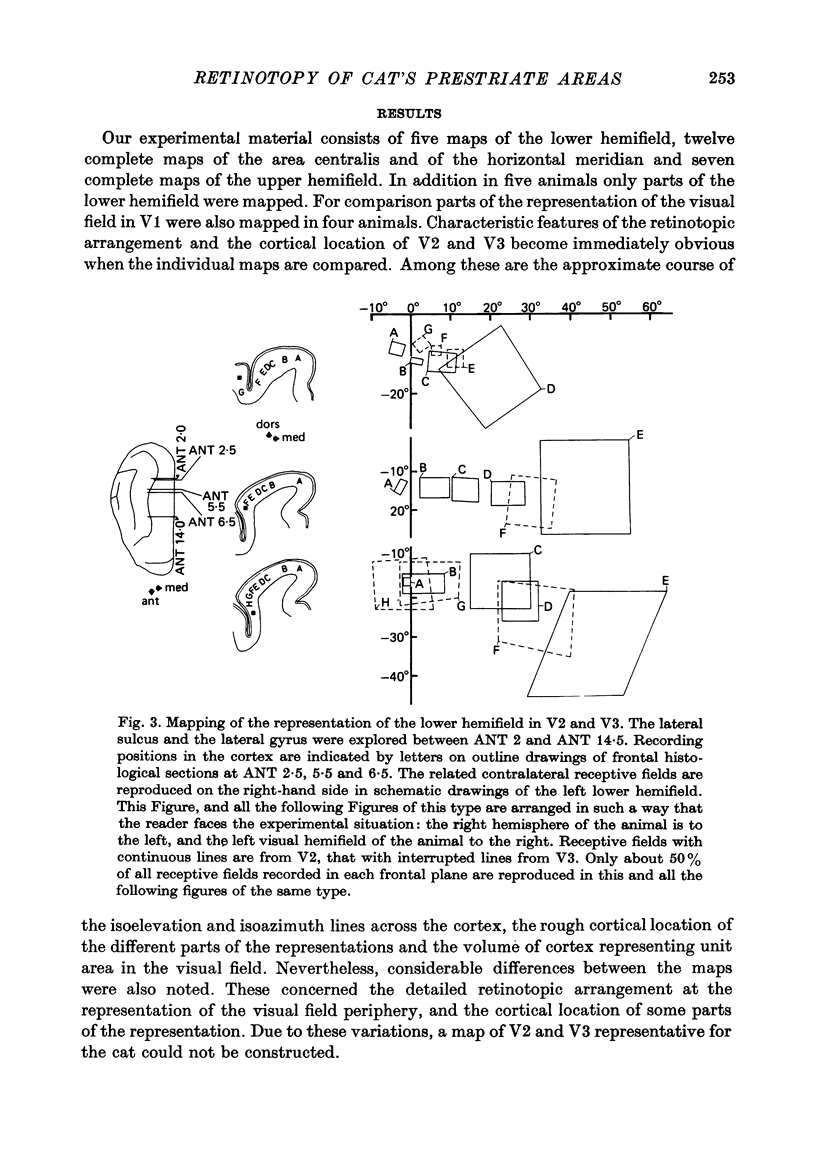
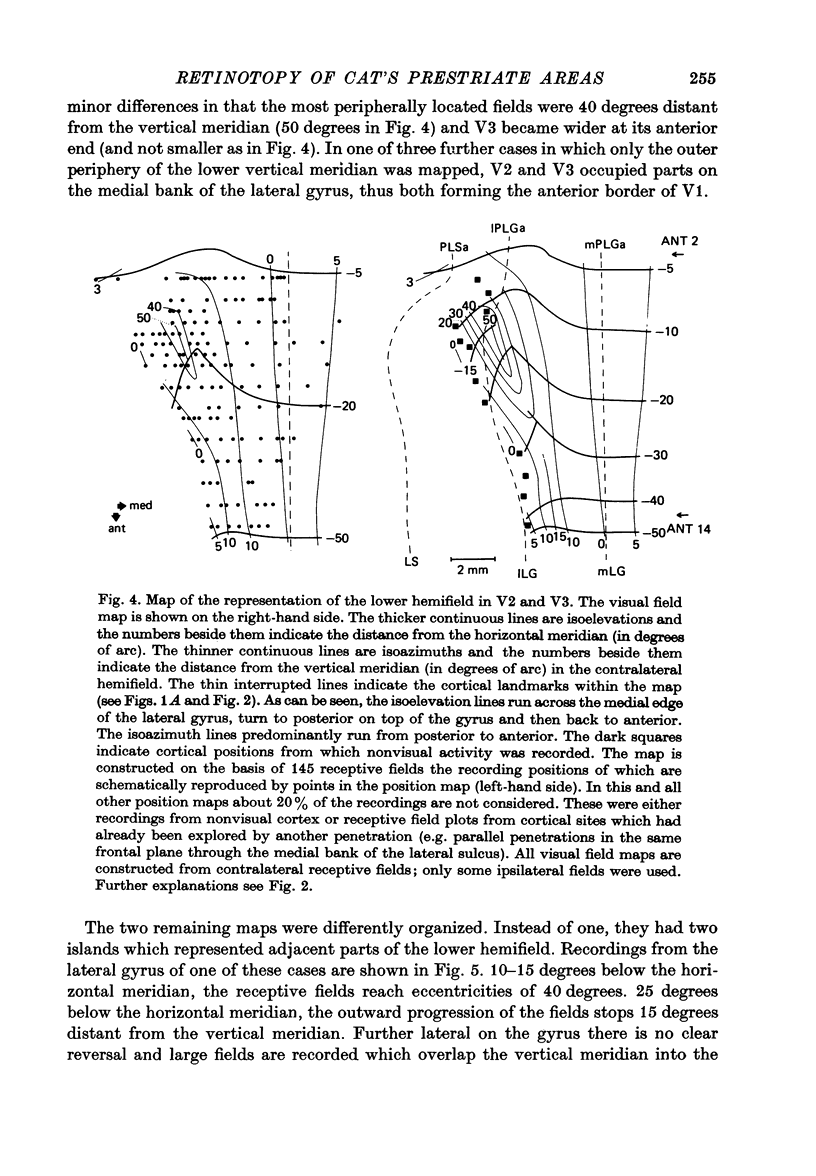
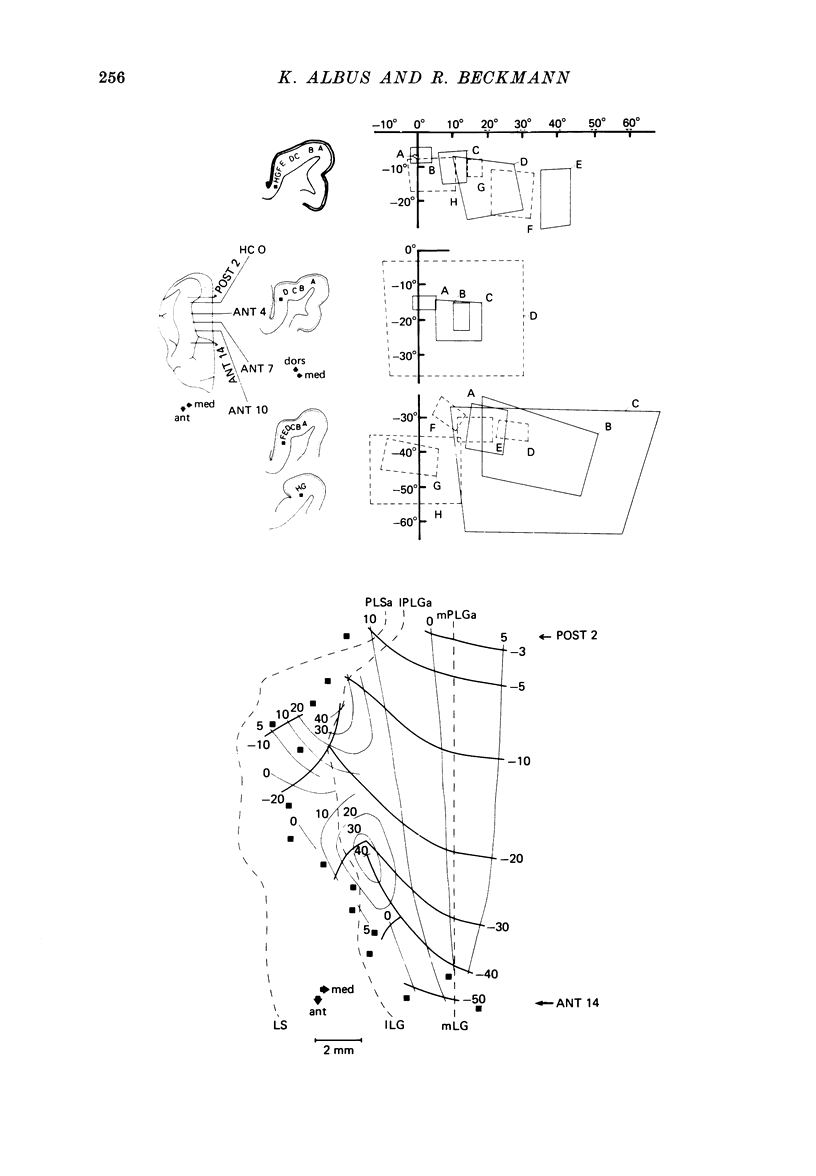
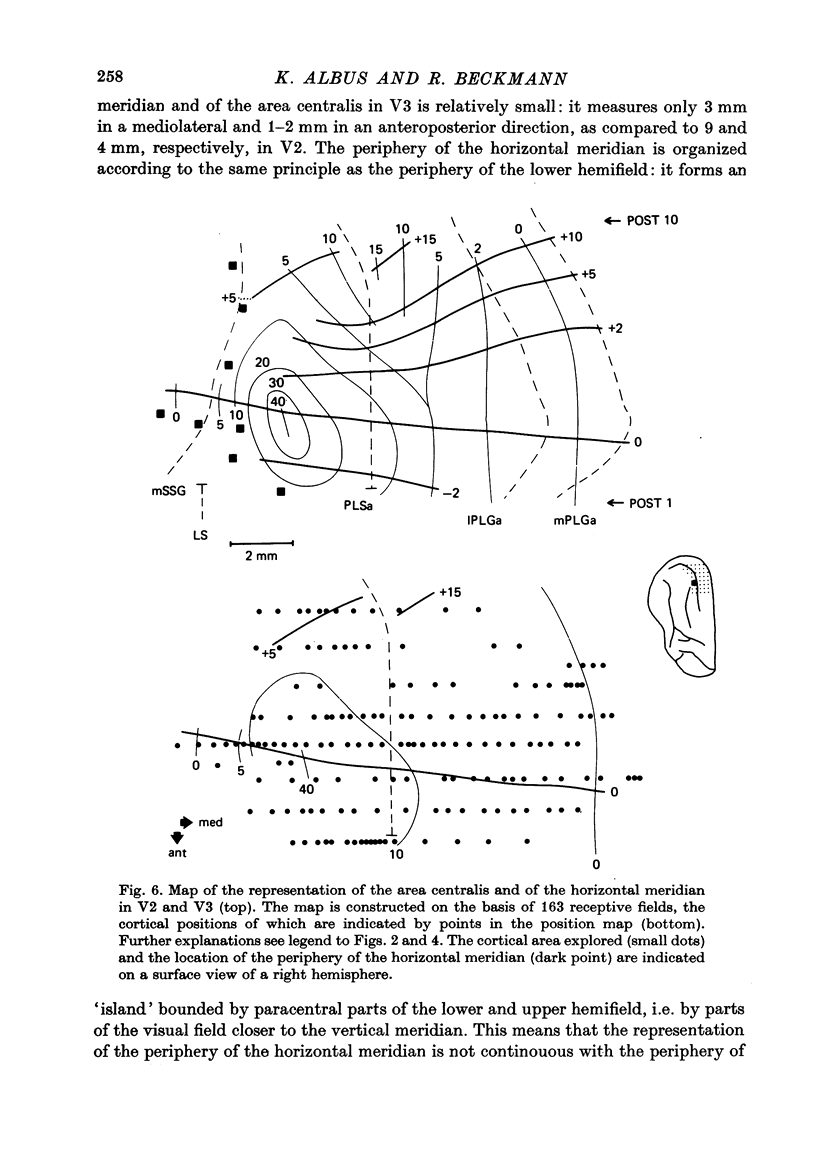
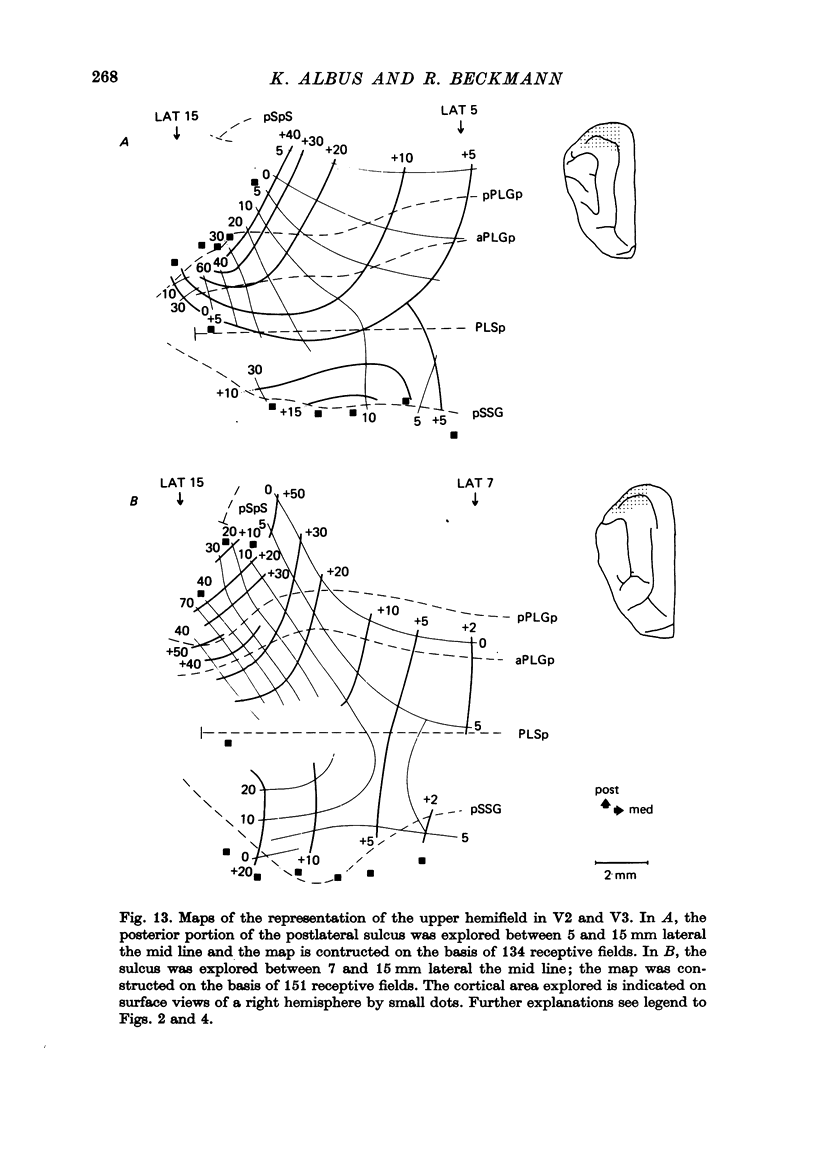
3. The detailed retinotopic arrangement of the visual field maps shows two characteristic features. First, the retinotopy at the V2/V3 border differs between lower and upper hemifield. In the lower hemifield the periphery of the fields is represented, whereas in the upper hemifield the border between the representations is formed by a sector running along the horizontal meridian about 5-10 degrees in the upper hemifield. Thus the lower field arrangement resembles that of rodents, and the upper field arrangement is similar to that of primates. Secondly, the periphery of a part of the visual field is not continuously represented, but forms patches or islands (Donaldson & Whitteridge, 1977). The islands are bounded by visual field representations closer to the vertical meridian. The way the visual field is represented at the border between V2 and V3 introduces discontinuities into the visual field maps: adjacent parts of the visual field are not represented adjacently in these two prestriate areas.
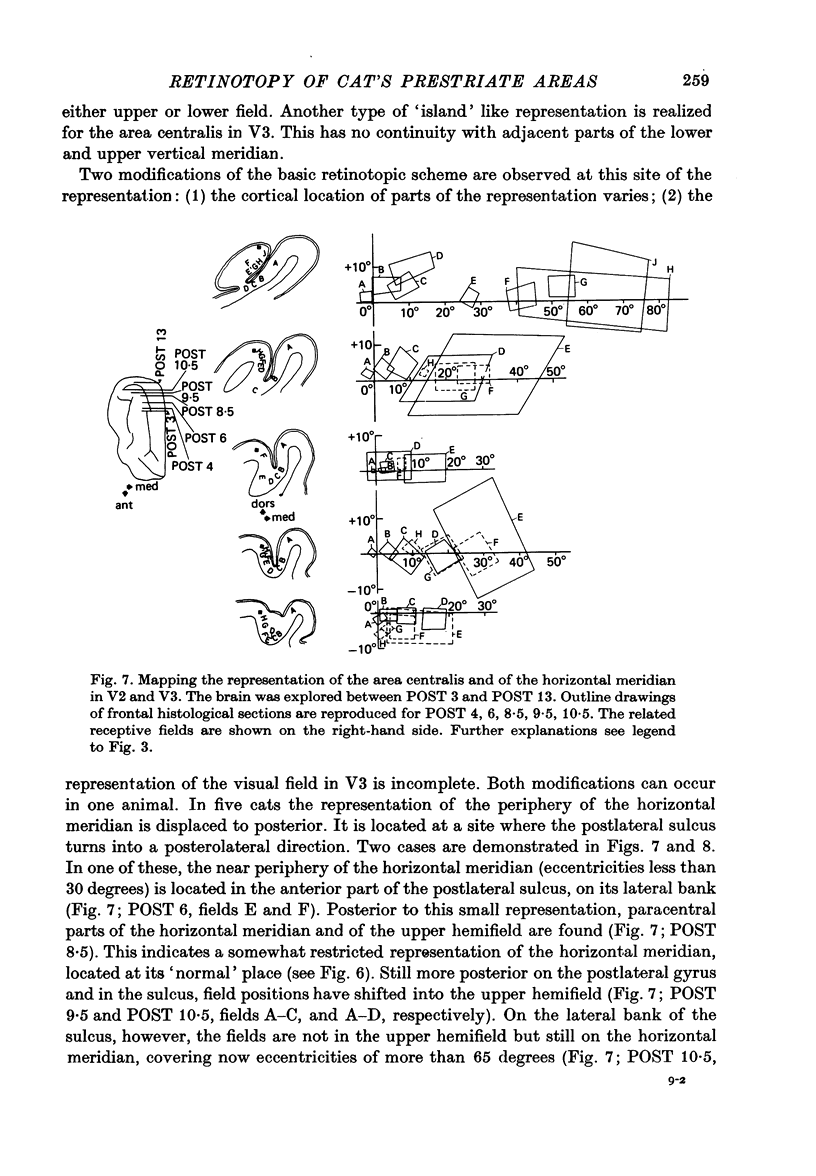
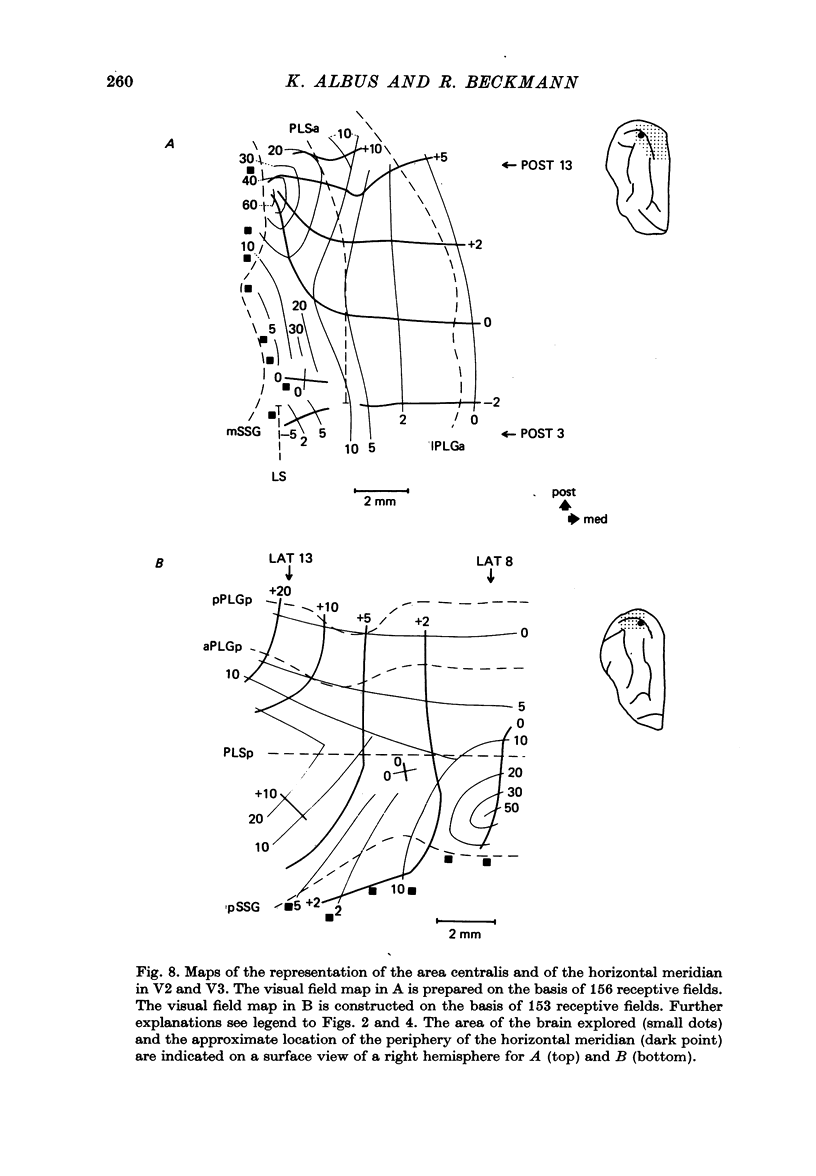
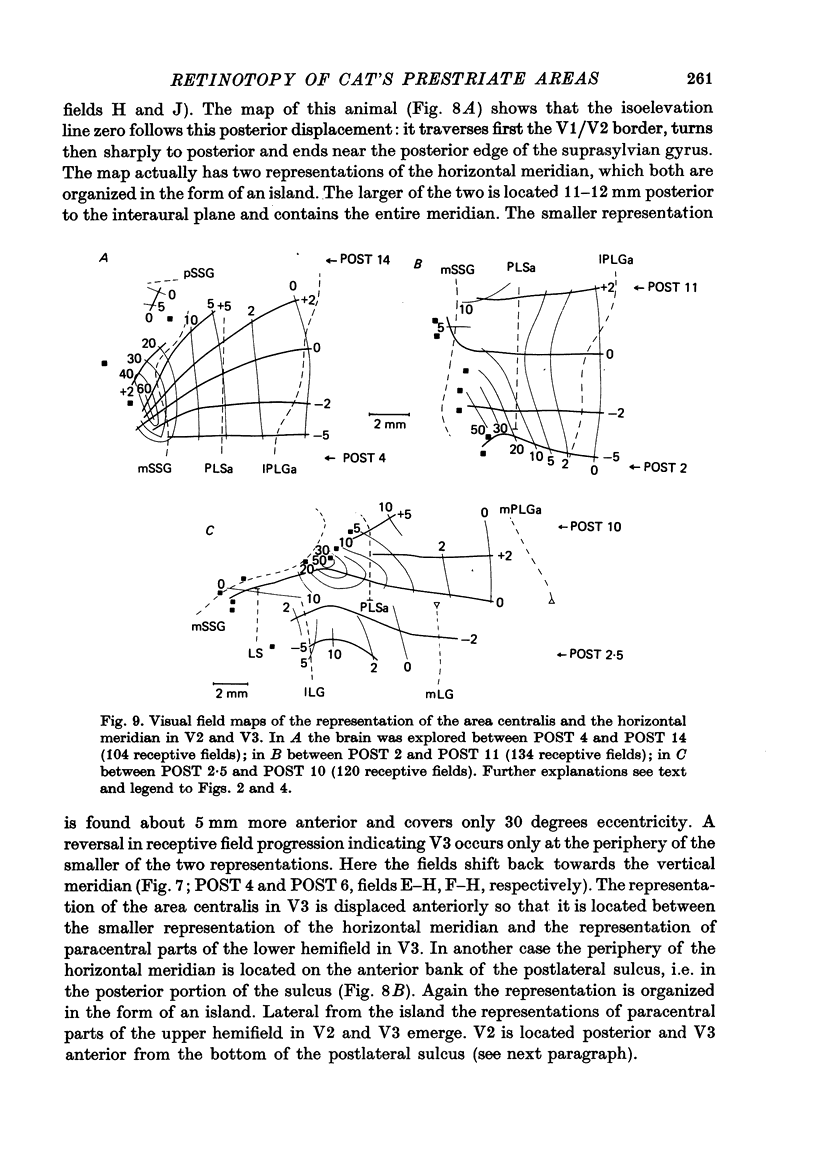
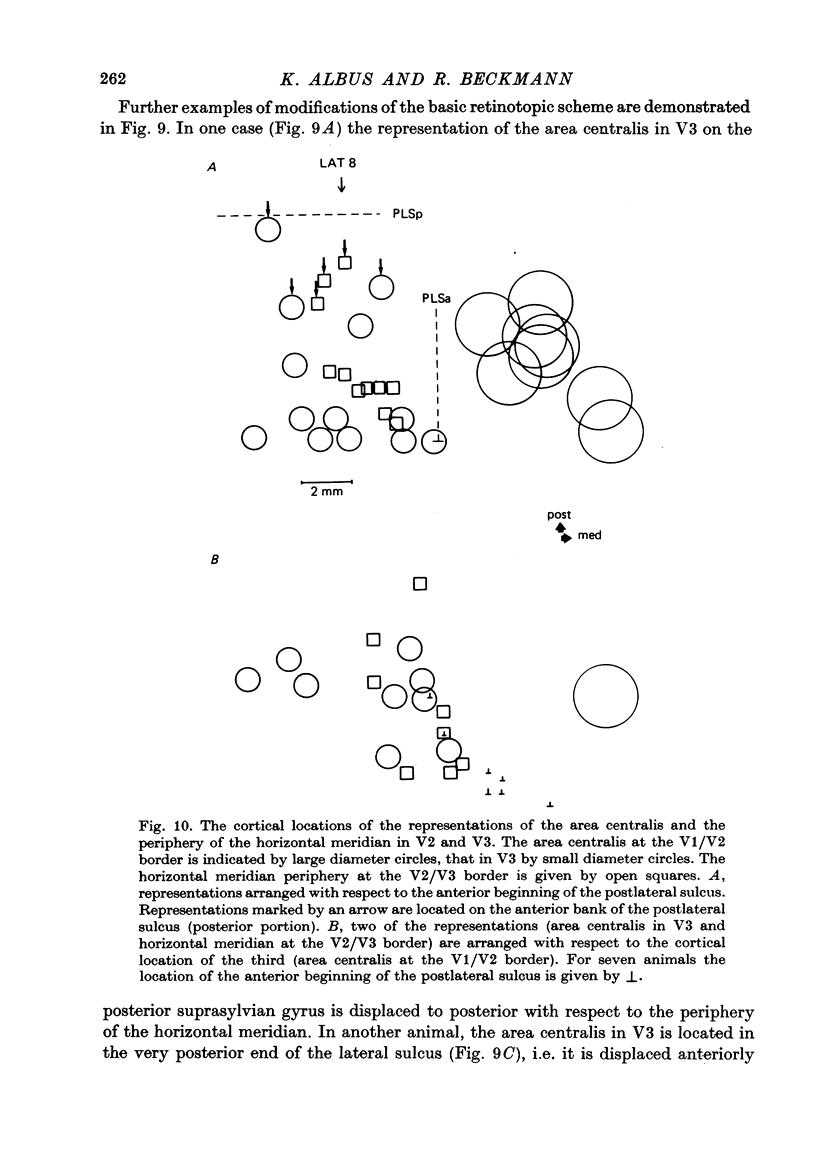
4. Cortical location and detailed retinotopic arrangement vary considerably from animal to animal, so that a representative map of V2 and V3 cannot be constructed. For example, the representation of the periphery of the horizontal meridian may be located either in the anterior portion of the postlateral sulcus or some mm more posteriorly, where the sulcus turns laterally. The representation of the area centralis in V3 is found either at the transition zone between lateral and postlateral sulcus, on the posterior suprasylvian gyrus, or in the posterior part of the postlateral sulcus.
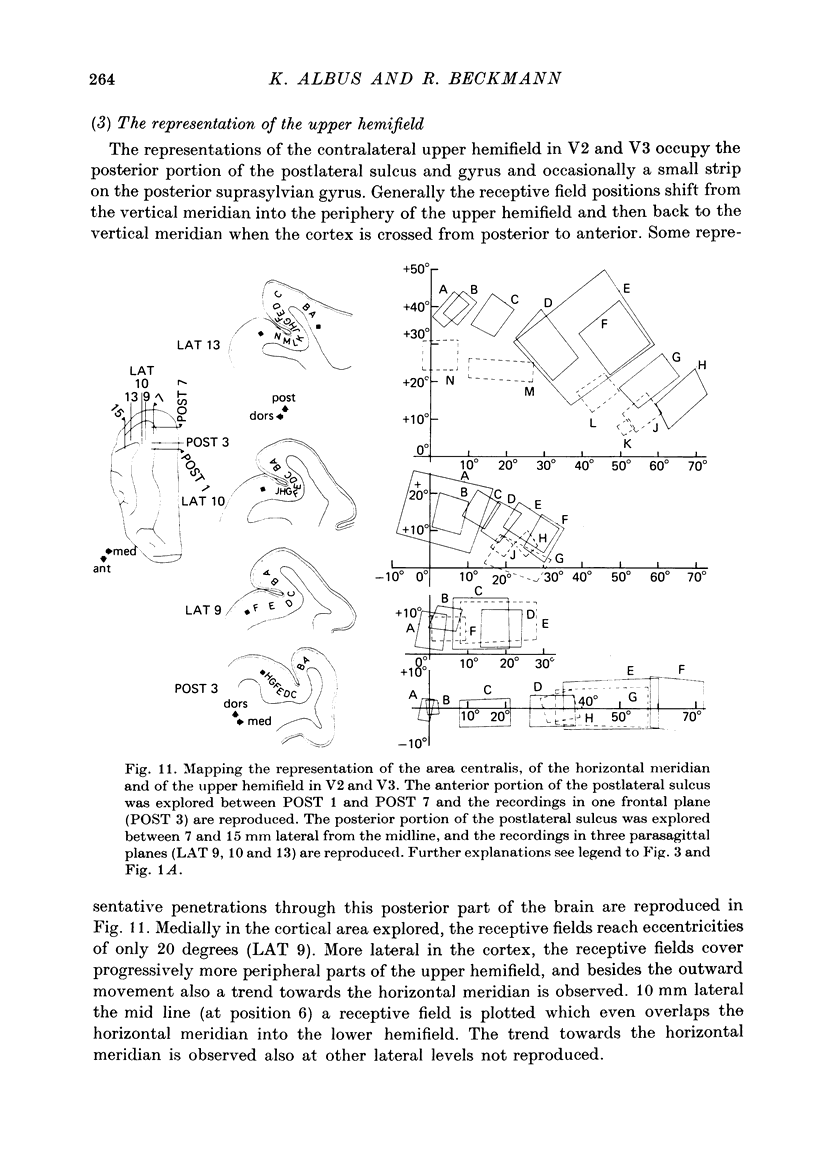
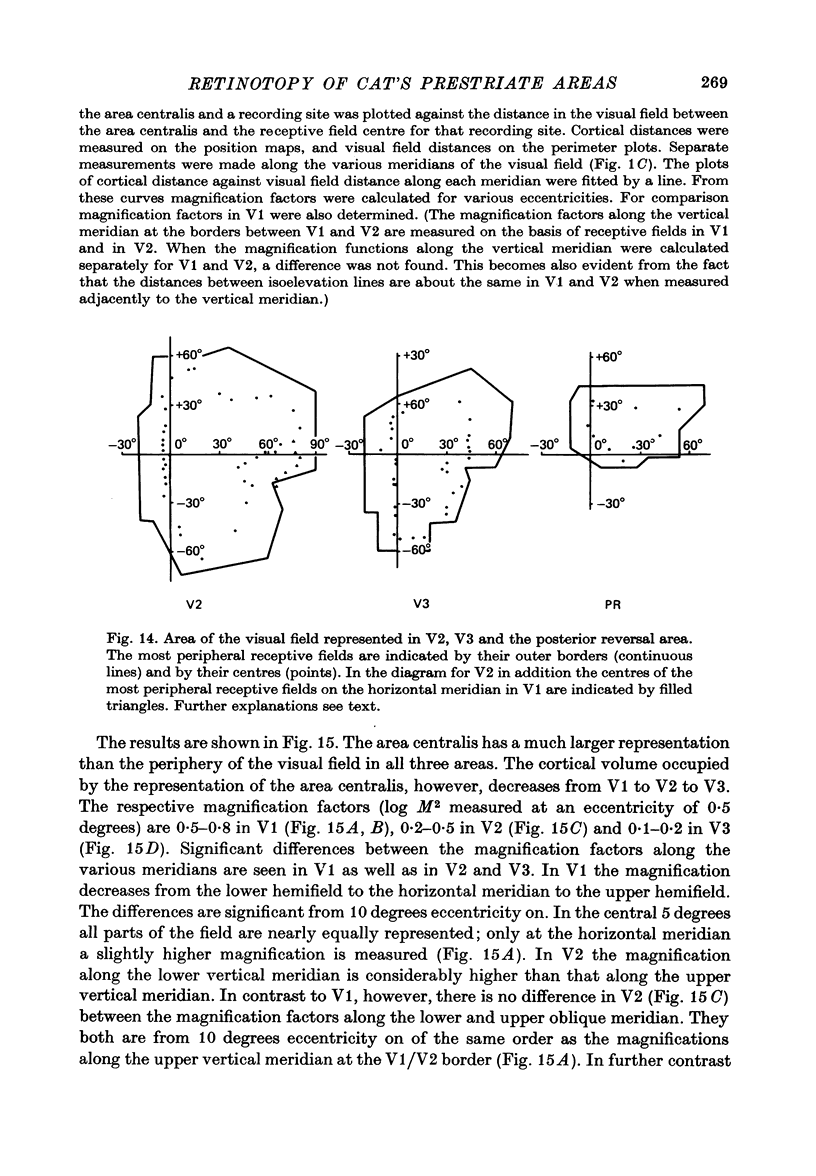
5. The entire hemifield is represented in V2 at least in some animals. In V3 the uppermost part of the vertical meridian seems not to be represented. In other animals only a restricted part of the contralateral visual field is represented in V2 or in V3. In these cases the receptive fields cover not more than 50 degrees out in the lower hemifield or on the horizontal meridian. In a few cases the periphery of the horizontal meridian and the upper hemifield are not at all represented in V3, or only in an incomplete manner.
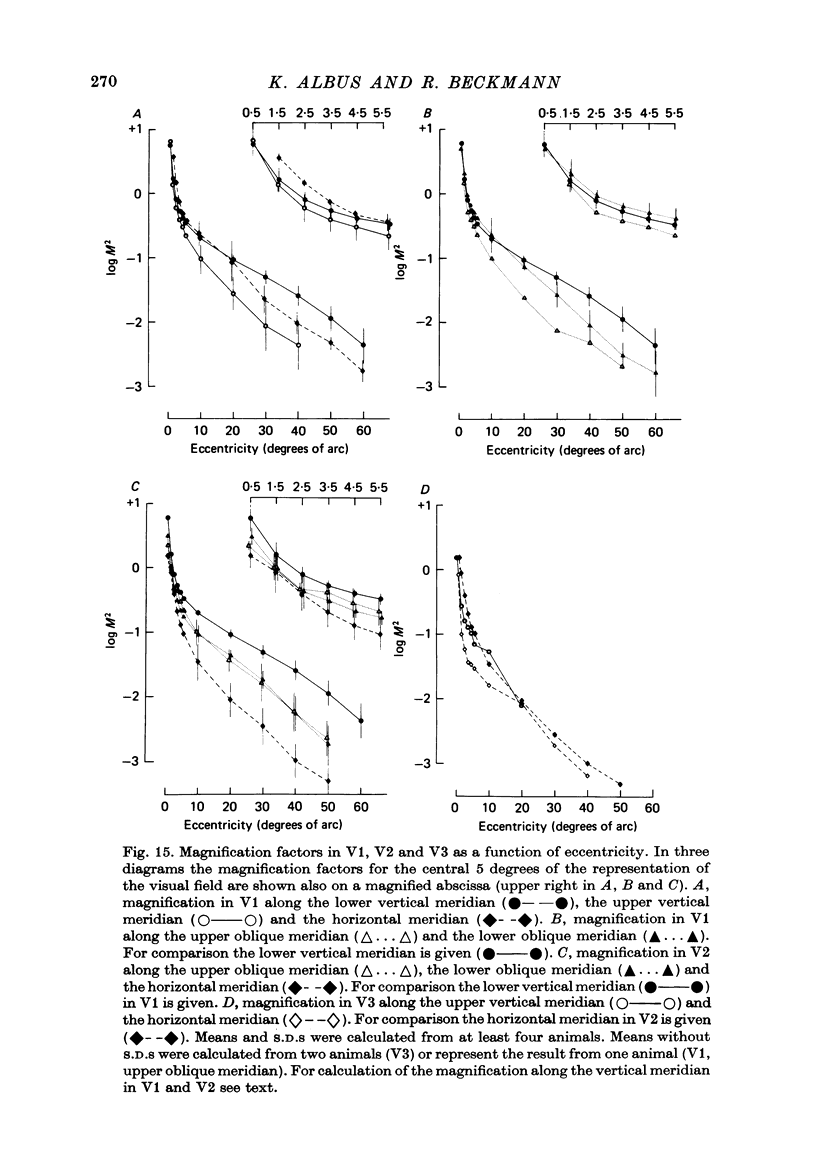
6. The magnification factors (Daniel & Whitteridge, 1961) become progressively smaller from V1 to V2 to V3. Hence cortical volume occupied decreases from V1 to V3. In V1 and in V2 the magnification is highest along the lower vertical meridian. In V2 the magnification along the horizontal meridian is the smallest, whereas in V1 the magnification decreases progressively from the lower vertical, to the horizontal and to the upper vertical meridian. The relationship between retinal ganglion cell densities and cortical magnification factors is discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. D., Forrester J. M. The projection of the rat's visual field on the cerebral cortex. Q J Exp Physiol Cogn Med Sci. 1968 Jul;53(3):327–336. doi: 10.1113/expphysiol.1968.sp001974. [DOI] [PubMed] [Google Scholar]
- Allman J. M., Kaas J. H. Representation of the visual field on the medial wall of occipital-parietal cortex in the owl monkey. Science. 1976 Feb 13;191(4227):572–575. doi: 10.1126/science.814619. [DOI] [PubMed] [Google Scholar]
- Allman J. M., Kaas J. H. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Res. 1975 Dec 26;100(3):473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Allman J. M., Kaas J. H. The organization of the second visual area (V II) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974 Aug 16;76(2):247–265. doi: 10.1016/0006-8993(74)90458-2. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge M., Bingle A., Seneviratne K. N., Whitteridge D. A map of the visual cortex in the cat. J Physiol. 1967 Jul;191(2):116P–118P. [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson I. M., Whitteridge D. The nature of the boundary between cortical visual areas II and III in the cat. Proc R Soc Lond B Biol Sci. 1977 Dec 13;199(1136):445–462. doi: 10.1098/rspb.1977.0153. [DOI] [PubMed] [Google Scholar]
- Dräger U. C. Receptive fields of single cells and topography in mouse visual cortex. J Comp Neurol. 1975 Apr 1;160(3):269–290. doi: 10.1002/cne.901600302. [DOI] [PubMed] [Google Scholar]
- Essen D. C., Zeki S. M. The topographic organization of rhesus monkey prestriate cortex. J Physiol. 1978 Apr;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath C. J., Jones E. G. The anatomical organization of the suprasylvian gyrus of the cat. Ergeb Anat Entwicklungsgesch. 1971;45(3):3–64. [PubMed] [Google Scholar]
- Hughes A. A quantitative analysis of the cat retinal ganglion cell topography. J Comp Neurol. 1975 Sep;163(1):107–128. doi: 10.1002/cne.901630107. [DOI] [PubMed] [Google Scholar]
- Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:33–159. doi: 10.1007/BF00142518. [DOI] [PubMed] [Google Scholar]
- Kalia M., Whitteridge D. The visual areas in the splenial sulcus of the cat. J Physiol. 1973 Jul;232(2):275–283. doi: 10.1113/jphysiol.1973.sp010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. L. Representation of the cochlea within the anterior auditory field (AAF) of the cat. Brain Res. 1977 Jul 22;130(3):447–467. doi: 10.1016/0006-8993(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Sur M., Lin C. S. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in "SI" in the owl monkey (Aotus trivirgatus). J Comp Neurol. 1978 Sep 1;181(1):41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C., Tusa R. J. The retinotopic organization of lateral suprasylvian visual areas in the cat. J Comp Neurol. 1978 Jan 15;177(2):237–256. doi: 10.1002/cne.901770205. [DOI] [PubMed] [Google Scholar]
- Sanides F., Hoffmann J. Cyto- and myeloarchitecture of the visual cortex of the cat and of the surrounding integration cortices. J Hirnforsch. 1969;11(1):79–104. [PubMed] [Google Scholar]
- Sprague J. M., Levy J., DiBerardino A., Berlucchi G. Visual cortical areas mediating form discrimination in the cat. J Comp Neurol. 1977 Apr 1;172(3):441–488. doi: 10.1002/cne.901720305. [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965 Jun;124(3):337–352. doi: 10.1002/cne.901240305. [DOI] [PubMed] [Google Scholar]
- Woolsey C. N. Comparative studies on cortical representation of vision. Vision Res. 1971;Suppl 3:365–382. doi: 10.1016/0042-6989(71)90051-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969 Jul;14(2):271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Simultaneous anatomical demonstration of the representation of the vertical and horizontal meridians in areas V2 and V3 of rhesus monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Feb 11;195(1121):517–523. doi: 10.1098/rspb.1977.0024. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. The functional organization of projections from striate to prestriate visual cortex in the rhesus monkey. Cold Spring Harb Symp Quant Biol. 1976;40:591–600. doi: 10.1101/sqb.1976.040.01.055. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol. 1978 Apr;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]


