Abstract
Passive administration of monoclonal antibodies (MAbs) to the capsular polysaccharide of Cryptococcus neoformans can alter the course of infection in mice. In a murine model of cryptococcal infection, immunoglobulin G1 (IgG1), IgG2a, and IgG2b switch variants of the anti-capsular 3E5 MAb prolong the survival of lethally infected mice, whereas the 3E5 IgG3 MAb does not protect and in some cases enhances infection, shortening the life spans of infected mice. We examined the role of complement component C3 in Ab-mediated protection by determining the efficacy of the four mouse IgG subclasses against C. neoformans in mice genetically deficient in factor C3 as well as mice acutely depleted of C3. Similar to other complement-deficient animal models, C3−/− mice and mice depleted of C3 by cobra venom factor were more susceptible to C. neoformans infection than control mice, providing further evidence that complement is important in the host defense against the fungus. In the absence of C3, all IgG isotypes prolonged the lives of mice infected with C. neoformans, indicating that protection by IgG does not require the complement pathways. Furthermore, we observed protection with IgG3 in the complement-deficient mice, suggesting that complement is involved in the lack of protection observed with IgG3 in other mouse models.
Cryptococcus neoformans is an opportunistic fungus that causes life-threatening meningitis in 6 to 8% of people with AIDS in the United States and in close to 30% of human immunodeficiency virus-infected individuals in Africa (5; H. Taelman, J. Clerinx, A. Kagame, J. Batungwanayo, A. Nyirabareja, and J. Bogaerts, Letter, Lancet 338:761, 1991). C. neoformans possesses an anti-phagocytic polysaccharide capsule, which is primarily composed of glucuronoxylomannan (GXM). In addition to inhibiting phagocytosis of the fungus (25), the polysaccharide capsule inhibits leukocyte migration (10), diminishes antibody (Ab) responses (28, 37, 46), alters cytokine secretion (42), and enhances replication of human immunodeficiency virus in vitro (40). Current therapy for cryptococcosis is inadequate because 10 to 20% of patients treated with antifungal drugs die from the disease. Individuals with cryptococcal meningitis who survive the initial illness must be maintained on lifelong antifungal therapy to prevent recurrence of infection (59). Because of these therapeutic shortcomings, passive administration of monoclonal Abs (MAbs) to GXM is being tested for prevention and as adjunct therapy against cryptococcal disease. A murine immunoglobulin G1 (IgG1) MAb is currently in a phase I clinical trial in AIDS patients with chronic cryptococcal meningitis (3).
Numerous animal studies have demonstrated that cell-mediated immunity and the complement system are vital in the natural host defense against encapsulated C. neoformans, whereas the role of humoral immunity is less clear (22, 23, 30, 31, 36). The complement system consists of a group of proteins that, when activated in a cascade, can provide defense against microorganisms by releasing factors that (i) recruit inflammatory cells, (ii) opsonize microbes, and (iii) can kill some pathogens by formation of the membrane attack complex. The complement system can be activated by two distinct pathways—the classical pathway and the alternate pathway—that converge at C3, the third component of complement. The classical pathway requires antigen-Ab complexes for activation, while the alternate pathway is part of the innate immune system and can be activated directly by microbial surfaces. Because little Ab is generated against GXM during infection (21, 28, 37), complement deposition on the surface of encapsulated C. neoformans is largely the result of alternate-pathway activation (22, 26, 27). Incubation of cryptococci with serum results in the rapid deposition of iC3b fragments on the polysaccharide capsule (24, 25), and complement alone can facilitate the opsonization and phagocytosis of encapsulated C. neoformans in vitro (7). Complement-deficient animals are more susceptible to C. neoformans infection (4, 8, 19, 44). Studies with cells expressing heterologous complement receptors indicate that complement receptor 1 (CR1), CR3, and CR4 are each capable of interacting with C. neoformans opsonized with C3 (32).
We (34, 39, 55, 56) and others (12, 18, 45) have reported that passive administration of IgM, IgG1, IgG2a, and IgG2b MAbs to GXM can prolong the survival of mice lethally infected with C. neoformans. For convenience, we have called this effect “protection.” However, administration of IgG3 MAbs with an identical variable region does not prolong survival and in some situations can enhance disease (55-57). MAbs of all isotypes accelerate GXM clearance from serum despite differences in protective efficacy (29). Both we (34, 55, 57) and Dromer et al. (11, 12) have shown that passively administered IgG MAbs to GXM can prolong or shorten the lives of mice that are genetically deficient in the C5 component of complement in the same isotype-dependent manner. C5 contributes to formation of the membrane attack complex, stimulating inflammation and recruiting phagocytes (9). However, the membrane attack complex does not penetrate the cryptococcal cell wall, so it is perhaps not surprising that C5 does not to play a role in Ab-mediated resistance to C. neoformans.
In this study, therefore, we have explored the role of C3, which is more proximal and central to both pathways, in passive Ab immunization against cryptococcal infection by analyzing the ability of MAbs of all of the IgG isotypes to protect both C3−/− mice and mice acutely depleted of C3 with cobra venom factor (CVF). Host susceptibility to C. neoformans significantly increased in the absence of C3. However, all IgG isotypes, including IgG3, protected against cryptococcal infection in the absence of C3, indicating that protection with passive Ab does not require complement and that the presence of C3 is, at least in part, necessary for the lack of protection seen with IgG3 in a variety of mouse models (1, 34, 55-58).
MATERIALS AND METHODS
Mice.
C3-deficient (C3−/−) mice were constructed by homologous recombination in J1 embryonic stem cells, a cell line established from a 129Sv mouse embryo, as described previously (52). The targeting vector disrupted the C3 gene. The embryonic stem cell was then microinjected into C57BL/6J blastocysts and transferred to the uteri of pseudopregnant female mice. Brother-sister matings of heterozygous offspring generated 129Sv × C57BL/6J F2 mice homozygous for the mutant C3 allele.
Small breeding colonies were maintained in a pathogen-free gnotobiotic facility within the Animal Care Institute at Albert Einstein College of Medicine. Prior to experimentation, the mice were transferred and maintained in a pathogen-free barrier facility. C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and maintained in the same pathogen-free barrier facility.
C. neoformans.
Strain 24067 (serotype D) was obtained from the American Type Culture Collection (Manassas, Va.). This strain has been used in all our previous studies of Ab-mediated protection against C. neoformans (55-58) and is a standard strain. The organisms were stored in culture medium with 50% sterile glycerol at −80°C. When ready for use, 50 μl of stored culture was inoculated into Sabouraud's dextrose broth (Difco Laboratories, Detroit, Mich.) and grown in a rotary shaker at 37°C for 24 h. The organisms were then washed three times with cold phosphate-buffered saline, and the size of the inoculum was determined by counting in a hemocytometer and confirmed by scoring CFU plated on Sabouraud's dextrose agar plates at several dilutions.
MAbs.
The 3E5 IgG3 hybridoma cell line was made by the fusion of the NSO mouse myeloma to splenic B cells from mice immunized with GXM conjugated to tetanus toxoid (2). IgG1, IgG2a, and IgG2b switch variants of 3E5 were generated by in vitro isotype switching (55, 57). Sequence data confirmed that the V regions of the switch variants were all identical to the 3E5 IgG3 V region, and all isotypes bound to GXM as shown by enzyme-linked immunosorbent assay (ELISA) (55). Ascites fluid was obtained by intraperitoneal (i.p.) injection of 5 × 106 hybridoma cells into Pristane-primed (Sigma Chemical Co., St. Louis, Mo.) SCID mice. The ascites fluid was collected and centrifuged to remove cells. Lipids and cell debris were removed with Cleanascite HC (LigoChem, Fairfield, N.J.), and the ascites fluid was sterilized by successive passage through 0.8-, 0.4-, and 0.2-μm-pore-size filters. The Ab concentration was quantitated by ELISA relative to isotype-matched standards of known concentration. The ascites fluid was stored at 4°C and was then filtered and quantitated again prior to animal experiments to establish sterility and the Ab concentration. In addition, the titer of each of the isotypes was determined by ELISA for its ability to bind to GXM, and the titers were comparable to those that had been used in previous studies.
C3 depletion.
To determine the length of time that CVF depletes C3 from mice, 14 C57BL/6J mice were injected i.p. with two 5-U doses of CVF (Quidel, San Diego, Calif.) 4 h apart. On days 1 through 7 postinjection, different pairs of mice were bled, along with two control mice not treated with CVF. The sera were separated from the blood and stored at −80°C. After all of the mice were bled, the sera were thawed and compared by ELISA using the MAb RmC11H9 (Cedarlane Laboratories, Hornby, Ontario, Canada) for the presence of C3.
Murine infection.
Groups of 8 to 11 C3−/− mice that were 8 to 12 weeks old were injected i.p. with SCID ascites fluid containing 1.0 mg of either 3E5 IgG1, 3E5 IgG2a, 3E5 IgG2b, or 3E5 IgG3 MAb or with 1.0 ml of SCID ascites fluid made from NSO, the nonproducing mouse myeloma cell line that was the fusion partner of 3E5. Twenty-four hours later, the mice were infected intravenously (i.v.) with ∼5 × 105 C. neoformans cells in 0.2 ml of phosphate-buffered saline, and deaths were recorded daily. To test the effects of IgM treatment in C3−/− mice, C3−/− mice were treated i.p. with SCID ascites fluid containing 1.0 mg of 12A1 IgM 30 min prior to i.p. infection with 106 C. neoformans cells, as previously described (39). We chose this route of infection for these experiments because IgM is protective against i.p. infection by C. neoformans but is not effective in prolonging survival when mice are infected i.v. (48).
C57BL/6J mice were injected i.p. with two 5-U doses of CVF 4 h apart to deplete the animals of C3. Twenty-four hours later, the mice were treated with either 1.0 mg of IgG2a, IgG2b, or IgG3 or 1.0 ml of NSO SCID ascites fluid and infected i.v. with 105 C. neoformans cells 48 h after CVF injection. The mice were injected with 5 U of CVF every fifth day until day 30 and were killed 50 days after infection.
Organ histology and CFU.
Groups of four C3−/− mice were given either 1.0 mg of IgG MAb or 1.0 ml of control NSO SCID ascites fluid 24 h prior to i.v. infection with 106 CFU of C. neoformans. In a separate experiment, two groups of four C3−/− mice were treated with either 12A1 IgM or control ascites fluid 30 min prior to i.p. infection with 106 C. neoformans cells. Organ histology was examined as described previously (13). The mice were euthanized by cervical dislocation 2 days postinfection. The left lung, accessory lobe of the liver, caudal third of the spleen, and left hemisphere of the brain were removed and fixed in 10% buffered formalin (Sigma) and embedded in paraffin. Sections stained with hematoxylin and eosin and with mucicarmine were examined by light microscopy (one to three sections per organ). The right hemispheres of the brains, right lungs, spleens (except for the caudal one-third), and livers (except for the accessory lobe) were removed, homogenized, diluted in phosphate-buffered saline, and plated on Sabouraud's dextrose agar to determine the number of CFU per organ as described previously (13).
Statistics.
Data were analyzed with Stat View statistical software (SAS Institute, Cary, N.C.). CFU data were evaluated using the Kruskal-Wallis test for nonparametric data. Kaplan-Meier analysis was applied to the survival experiments, and statistical significance was determined by the log rank (Mantel-Cox) test. A P value of less than 0.05 was considered statistically significant.
RESULTS
IgG MAbs protect against cryptococcal infection in mice in the absence of C3.
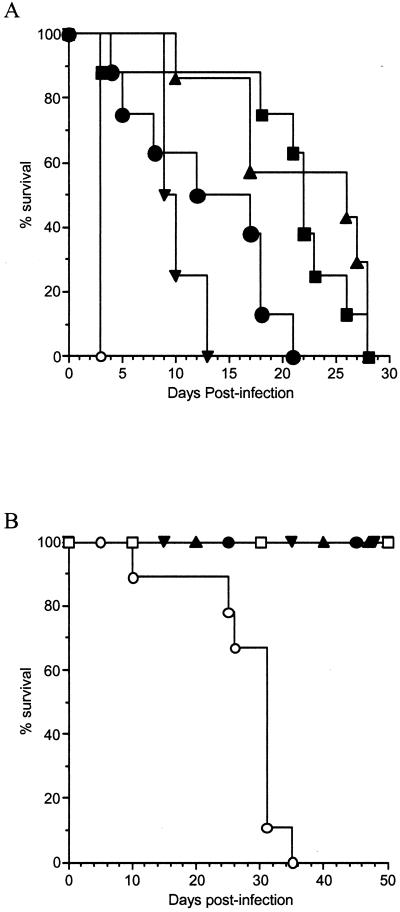
We have previously reported that the IgG1, IgG2a, and IgG2b members of the 3E5 family of isotype-switched MAbs prolong the lives of mice lethally infected i.v. with C. neoformans. In contrast, the IgG3 member of this family does not prolong the lives of such mice and can even enhance infection (55-57). To determine the role of C3 in passive immunization against cryptococcal infection, C3−/− mice were injected i.p. with either 3E5 IgG1, IgG2a, IgG2b, or IgG3 or control NSO SCID ascites fluid prior to i.v. infection with 105 cryptococci (Fig. 1A). Mice given IgG1, IgG2a, IgG2b, and IgG3 lived longer than control animals. This demonstrated that all IgG isotypes, including IgG3, protected against infection in the absence of C3 (P < 0.0009). We repeated this experiment using an inoculum of 106 organisms and obtained similar results. All IgG isotypes, including IgG3, were protective (median survival times [in days] were as follows: IgG1, 5 ± 0.8; IgG2a, 5 ± 0.4; IgG2b, 4 ± 0.8; IgG3, 4 ± 0.5) relative to controls (2 ± 0.5; P < 0.001) at the higher inoculum. Although all of the isotypes were protective in the C3-deficient mice, the only statistically significant difference among the isotypes was that IgG1 was more protective than IgG3 at both inocula (P < 0.023). These same Ab preparations gave the expected results in wild-type, complement-sufficient C57BL/6J mice, i.e., IgG3 was not protective while IgG1, IgG2b, and IgG2a were protective, as previously reported (1).
FIG. 1.
Passive Ab protects mice lacking C3. (A) Survival of C3−/− mice infected with C. neoformans after i.p. administration of 1.0 mg of 3E5 IgG1 (▪), IgG2a (▴), IgG2b (▾), or IgG3 (•) anti-GXM SCID ascites fluid or 1.0 ml of control NSO (○) SCID ascites fluid 24 h prior to i.v. challenge with 5 × 105 C. neoformans cells. There were 8 to 11 mice in each group. The median survival times (± standard deviations) for the IgG1, IgG2a, IgG2b, IgG3, and NSO groups were 22 ± 8, 27 ± 7, 10 ± 2, 14 ± 7, and 3 ± 0 days, respectively. All isotypes prolonged animal survival significantly (P < 0.0009). (B) Survival of C. neoformans-infected C57BL/6J mice depleted of C3 by CVF. Twenty-four hours after CVF injection, the mice were given 1.0 mg of 3E5 IgG2a (▴), IgG2b (▾), or IgG3 (•) anti-GXM SCID ascites fluid or 1.0 ml of control NSO (○) SCID ascites fluid 24 h prior to i.v. challenge with 105 yeast cells. NSO-treated mice not given CVF are also shown (□). There were 9 to 11 mice in each group. After 35 days, all control mice treated with CVF had died (median survival time, 31 ± 7 days), whereas there were no deaths among animals that received Ab or control mice that did not receive CVF (P < 0.002).
In limited studies of C3-sufficient mice of the 129 × C57BL/6J mixed background, IgG3 was not protective (data not shown), as we have observed in other mouse strains. However, we wished to confirm the observations made in the 129 × C57BL/6J C3−/− mice that IgG3 was protective in the absence of C3 by using a mouse strain in which we had repeatedly shown that IgG3 was not protective (1, 56, 57). C57BL/6J mice were therefore acutely depleted of C3 by CVF. CVF forms a stable C3 convertase upon binding to serum components and rapidly cleaves all circulating C3, resulting in C3 depletion (51). To determine the duration of C3 depletion by CVF, mice were injected with CVF. Pairs of mice were bled each day, and the amount of C3 in their sera was determined by ELISA. As reported previously (17), 10 U of CVF depleted C3 from C57BL/6J mice for 5 days. On day 6 postinjection, there was a rebound effect before C3 returned to preinjection levels on day 7 (data not shown).
Using this information, C57BL/6J mice were depleted of C3 by CVF treatment every fifth day. Twenty-fours hours after their initial CVF treatment, the mice were treated with IgG2a, IgG2b, or IgG3 MAb to GXM or NSO SCID ascites fluid and then infected with C. neoformans 24 h later (Fig. 1B). Because the untreated C3−/− mice had died so quickly, we used one-fifth the number of CFU to infect the CVF-treated mice. The control (NSO) mice treated with CVF all died by day 35 after infection, while all of the mice that did not receive CVF were still alive by day 50, at which time the experiment was terminated. This result provides additional confirmation of the importance of complement, and particularly C3, in the host defense against cryptococcal disease. All of the CVF-treated mice that had received MAbs were alive 50 days after infection. No morbidity was observed in these mice more than 2 weeks after the last of the CVF-treated control mice had died. As with the C3−/− mice, all 3E5 IgG isotypes were protective (P < 0.002), demonstrating that IgG MAb administration prolonged survival despite the absence of C3. Since IgG3 is not protective in C57BL/6J mice (56), we did not expect the IgG3 CVF-treated C57BL/6J mice to survive longer than the controls. However, the fact that they were still alive after 50 days is consistent with the similar observation with the C3−/− mice and suggests that C3 plays a role in the inability of IgG3 to protect against infection.
Effects of IgG MAbs on the number of CFU and histopathology of the organs of C3-deficient mice.
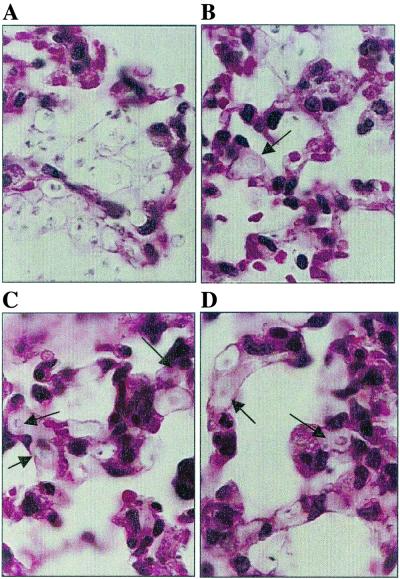
The number of CFU and the histopathology of the organs of C3−/− mice were examined. Because the control mice died on day 3, all mice were studied 2 days after infection with C. neoformans. Mice treated with each of the IgG isotypes had a lower fungal burden in the lung than control mice at this stage of infection (Table 1). Histological examination revealed that the lungs of mice that received control ascites fluid had large collections of extracellular yeast but no intracellular yeast, and there were few inflammatory cells (Fig. 2A). Mucicarmine-stained sections revealed that alveolar septae contained extensive amounts of capsular polysaccharide (data not shown). In C3−/− mice treated with 3E5 MAbs of all IgG isotypes, intracellular yeast cells were common inside macrophages, and fewer yeast cells were present per infectious focus in most mice (Fig. 2B to D), consistent with the CFU determinations (Table 1). The difference in appearance between the lungs of control and MAb-treated mice was such that one of us (S.S.), when blinded, could distinguish between the two groups in 19 of 20 slides. As with the control mice, polysaccharide was seen in the alveolar septae (data not shown).
TABLE 1.
Numbers of CFU in organs of IgG-treated C3−/− mice 2 days after C. neoformans infectiona
| Ascites fluid | No. of CFU (104)
|
|||
|---|---|---|---|---|
| Lung | Liver | Spleen | Brain | |
| NSO | 180 ± 93b | 7 ± 3.0 | 16.0 ± 13.0 | 0.08 ± 0.06 |
| IgG1 | 27 ± 19c | 20 ± 6.0c | 1.7 ± 0.4c | 0.30 ± 0.17c |
| IgG2a | 66 ± 26c | 20 ± 6.2c | 2.0 ± 0.4c | 0.09 ± 0.04 |
| IgG2b | 48 ± 27c | 21 ± 8.0c | 0.7 ± 0.4c | 0.12 ± 0.07 |
| IgG3 | 43 ± 33c | 39 ± 10.0c | 2.7 ± 1.0c | 0.23 ± 0.10c |
Groups of four mice were given either 1.0 mg of MAb or 1.0 ml of NSO ascites fluid 24 h prior to infection with 106 CFU of C. neoformans.
1 standard deviation.
P < 0.05 versus NSO.
FIG. 2.
Lung sections from C3−/− mice stained with hematoxylin and eosin 2 days after infection with C. neoformans following pretreatment with control ascites fluid (A) or IgG1 (B), IgG2a (C), or IgG3 (D). Four mice in each group were examined. MAb-treated animals reveal both intracellular (→) and extracellular yeast cells, whereas the NSO control animals show only extracellular organisms.
All MAb-treated C3−/− mice had significantly higher fungal burdens in the liver than control mice. This result suggested that IgG to GXM shifted the burden of organisms to the liver from the lung and spleen during the early stages of infection. Neither the MAb-treated nor the control mice had many inflammatory cells associated with the collections of extracellular organisms (data not shown). There were fewer CFU in the spleens of the mice treated with IgG MAb (Table 1), and consistent with this, there were fewer yeast cells in the occasional collections of organisms seen throughout the spleens of the MAb-treated mice than in the spleens of untreated mice (data not shown).
There were approximately three to four times more CFU in the brains of the IgG1- and IgG3-treated mice than in those of the controls. Consistent with the CFU data, histopathology revealed more infectious foci in the IgG1- and IgG3-treated mice than in the controls, but this was also true of the IgG2a- and IgG2b-treated mice. The lesions in the parenchymas of the Ab-treated mice were small, containing from one to six yeast cells that were located intra- or extracellularly and were not associated with inflammation.
Effects of IgM MAbs on cryptococcal infection in C3−/− mice.
Several murine IgM MAbs to GXM are protective against i.p. cryptococcal infection in various mouse strains (34, 39, 48). It was necessary to use an i.p. model because IgM MAbs are not protective against i.v. or intratracheal infection (48). Using the i.p. route of infection, we tested the ability of the protective IgM MAb 12A1 to prolong survival in lethally infected C3−/− mice. The median survival time in the control group was 10 ± 3 days, and the median survival time in the IgM-treated mice was 11 ± 3 days (P < 0.205). In contrast to the IgG isotypes, IgM administration did not prolong survival, suggesting that complement, and in particular C3, is necessary for IgM-mediated protection.
DISCUSSION
We have previously reported that passive immunization with the different isotypes of the 3E5 family of MAbs to GXM modulates infection with C. neoformans in various strains of mice (38, 55-57). Administration of 3E5 IgG1, IgG2a, or IgG2b before infection prolonged the life spans of lethally infected immunocompetent animals compared to controls. In contrast, 3E5 IgG3 did not protect against cryptococcal infection and in some cases even enhanced the infection (55-57). The observation that the passive administration of MAbs to GXM with different isotypes can lead to different disease outcomes stimulated us to investigate the mechanisms responsible for Ab-mediated modulation of infection. This has become especially important, as a MAb with the same specificity as the 3E5 family of Abs is being evaluated in AIDS patients with chronic cryptococcal infections.
The importance of complement in host defense against C. neoformans is suggested by several animal studies that have associated increased susceptibility to infection with complement depletion. CVF depletion of C3 makes guinea pigs (8) and DBA/1J, CeH/HeJ, and BALB/c mice more susceptible to cryptococcal infection than complement-sufficient animals (19). Ab that blocks CR3 shortens the life spans of CBA mice infected with C. neoformans (4). In this paper, we have shown that C57BL/6J mice depleted of C3 with CVF and C3−/− mice of mixed genetic background are also more susceptible to infection with C. neoformans. Apart from these in vivo observations, there is a large body of evidence from in vitro studies showing that complement components can serve as opsonins for C. neoformans, suggesting that complement contributes to the innate immunity to this organism (reviewed in reference 23).
While C3 is clearly important for natural resistance to cryptococcal infection, less is known about the interplay between protective and nonprotective passive Abs and complement. The 3E5 IgG1, IgG2a, and IgG2b MAbs used in these studies did prolong the life of A/J mice, which have defects that include a deficiency in C5 (53, 54), while 3E5 IgG3 decreased survival (34, 57). Since C3 is central to both complement pathways and could contribute directly to phagocytosis through complement receptors, we have now explored the contribution of C3 to MAb-mediated protection against C. neoformans in mice. Administration of IgG1, IgG2a, IgG2b, and IgG3, but not IgM, before lethal C. neoformans infection prolonged survival in C3-deficient mice. CVF-treated mice that received Ab also lived longer than the untreated controls. This observation contrasts with the earlier report that rabbit polyclonal serum was not protective in mice depleted of C3 by CVF (19). The difference between our results and those of the prior study could reflect variation in the amounts of Ab administered, the source of the Ab, or the fact that the rabbit serum is likely to have contained a high proportion of IgM, since the capsular polysaccharide preferentially elicits this isotype. Our results showing that IgM does not prolong survival in C3−/− mice may explain the different results if the rabbit serum was primarily IgM. Also, the two studies used different serotypes and strains of C. neoformans, different strains of mice, and different infection protocols.
The observation that the CVF-treated C57BL/6J mice given the 3E5 IgG3 isotype were protected relative to control mice is of particular interest. This isotype is either not protective or is disease enhancing in C57BL/6J mice, A/J mice, and FcRγ−/− mice that are fully backcrossed to C57BL/6J (34, 55, 57, 58). The lack of protection by IgG3 in wild-type mice is not due to something contaminating the MAb preparations, since we have seen this with many different preparations of 3E5 IgG3 and with different IgG3 anti-GXM Abs (34, 55-58). In addition, we have seen the same lack of protection when purified IgG3 is used in passive administration (1). We have proposed that the lack of protection and/or enhancement of infection is the result of IgG3 interaction with a unique IgG3 Fc receptor (FcR) (6, 58), which could lead to phagocytosis of the organism without killing and result in the intracellular growth and dissemination of the organism (55). It is also possible that IgG3 normally engages the putative IgG3 FcR and one of the complement receptors through the fixation of complement (32) and that this combination triggers events that prevent killing by phagocytic cells and increase dissemination of the organism. In the absence of complement, only the IgG3 FcR would be engaged, and that alone might lead to killing of the organism. This would require that the putative IgG3 FcR have special signaling properties that are different from those of the other IgG FcRs. Previously, the only other instance in which IgG3 was protective was in CD8−/− mice (56). Hence, it appears that IgG3-mediated lack of protection and enhancement of infection in some contexts requires C3 and/or CD8+ cells. This is in contrast to the role of C3 in the IgG3-mediated protection of mice lethally infected with Candida albicans (20). Although MAbs can both protect and enhance candida infections, C3 is required for IgG3 to prolong the life of mice infected with this organism (20). While these differences could be due to the distinct experimental conditions in the experiments reported by Han et al. (20) and those used in this study, they suggest that the interplay of Ab, complement, the capsule, and effector cells may be different for different organisms.
Histopathological examination of tissues and measurement of CFU in the organs suggest possible mechanisms for IgG-mediated protection in the C3-depleted mice. In C3−/− mice that had not received MAbs, virtually all C. neoformans cells were extracellular 2 days after infection. This finding is in sharp contrast to wild-type mice, which exhibit abundant intracellular and extracellular organisms in infected tissues (16). The absence of internalized C. neoformans cells in C3−/− mice suggests that complement is the primary opsonin or that it stimulates production of an opsonin in the absence of passive Ab. The observation that administration of IgG to C3−/− mice resulted in a large proportion of internalized organisms suggests that phagocytosis is one mechanism by which Ab treatment can lead to protection against cryptococcal disease. In addition, the presence of opsonizing Ab may enhance the cell-mediated response through improved antigen presentation as a consequence of more efficient phagocytosis and antigen presentation (41, 47, 49, 50).
IgG-treated mice had lower numbers of CFU in the organs and a shift in the fungal burden from the lungs and spleen to the liver. While it is possible that the rapid death of these mice was due to pneumonia, as has been suggested for C5-deficient mice (43), the amount of pulmonary destruction and the numbers of CFU were less than those seen in mice infected through the pulmonary route that survive for much longer periods of time (13, 15). CFU distribution in the organs corresponded with histopathological findings. In vitro studies have consistently shown that Ab-mediated phagocytosis can result in killing of fungus (13, 14, 35). We interpret the lower numbers of CFU in the organs in MAb-treated mice as indicative of Ab-mediated phagocytosis, resulting in the killing or arrest of growth of the fungus. IgG-mediated phagocytosis promotes the release of proinflammatory cytokines (50), suggesting an additional mechanism by which the presence of Ab may modify the course of infection. The proportionally larger numbers of CFU in the livers of Ab-treated mice suggest that liver phagocytes play an important role in the clearance of organisms complexed with Ab, as has been reported previously for GXM-Ab complexes (29). Overall, these results confirm that administration of IgG promotes phagocytosis in vivo and suggest that it facilitates the clearance of the organism. However, the three- to fourfold increase in CFU in the brains of IgG1- and IgG3-treated mice compared to the control is more difficult to understand, since these mice survived for a longer time. The histological examination also suggested that there were more organisms in the brains of IgG-treated mice than in those of untreated controls. This could reflect the dissemination of organisms that had been taken up by phagocytic cells, but there is little inflammation associated with the organisms in the brain. We do not have an explanation for this discrepancy between numbers of CFU and survival, although the literature includes several examples where the numbers of CFU in the organs do not correlate with the outcome (13, 15).
In contrast to the four IgG subclasses, administration of the IgM MAb 12A1 did not prolong survival in C3−/− mice, indicating that IgM requires complement for efficacy. Under these conditions, 12A1 is protective in C57BL/6J and A/J mice (39, 48). Similarly, a human IgM MAb was unable to mediate protection in CVF-depleted mice infected with C. neoformans (18). Taborda and Casadevall (48) demonstrated a prozone effect with IgM in complement-sufficient mice by studying many different inocula and Ab doses. Since IgM is so effective in fixing complement, it is likely that C3 is required for IgM-mediated protection. However, the lack of protection by IgM in the absence of C3 could be due to a shift in the prozone. A passively administered IgG1 MAb lost the ability to protect against C. neoformans infection at lower inocula (11). While we observed protection with all IgG isotypes at two different inocula, it is likely that the Ab dose, organism serotype and inoculum, and mouse strain all contribute to the outcome of passive immunization as a treatment for cryptococcal infection. In fact, the number of CFU in the organs was reduced in the IgM-treated C3-deficient animals compared to controls (data not shown), suggesting that the Ab was participating in the clearance of the fungus.
In summary, our findings show that IgG can prolong survival in the absence of C3. However, C3 appears to contribute to the lack of protection and/or enhancement observed upon IgG3 administration and is important for IgM-mediated protection. Our results also strongly support the importance of C3 in defense against C. neoformans infection by demonstrating increased susceptibility for C3−/− mice. Given the increasing interest in passive Ab therapy and the design of vaccines, which protect by eliciting protective Abs, our results highlight the need for additional research to dissect the mechanisms of Ab action. In addition, since fungemia can result in a decrease in C3 in some patients (33), these experiments suggest that Abs will be effective even in the setting of complement deficiency.
Acknowledgments
This research was supported by the following grants from the National Institutes of Health: T32 GM 07288 and AI 07506 (S. Shapiro), AI 01434 (D. O. Beenhouwer), AI 01341 (M. Feldmesser), AI36389 (M. C. Carroll), AI 33774, AI 13342, and HL 59842 (A. Casadevall), and AI 43937 (M. D. Scharff). M. D. Scharff is also supported by the Harry Eagle Chair provided by the Women's Division of the Albert Einstein College of Medicine.
We thank Jieru Zhang for technical support and Alvin Watford for assistance in animal breeding. We also thank Betty Diamond and Rena May for helpful comments and critical review of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 69:6445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall, A., J. Mukherjee, S. J. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 165:1086-1093. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross, C. E., H. L. Collins, and G. J. Bancroft. 1997. CR3-dependent phagocytosis by murine macrophages: different cytokines regulate ingestion of a defined CR3 ligand and complement-opsonized Cryptococcus neoformans. Immunology 91:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie, B. P., and A. Casadevall. 1994. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin. Infect. Dis. 19:1029-1033. [DOI] [PubMed] [Google Scholar]
- 6.Diamond, B., and D. E. Yelton. 1981. A new Fc receptor on mouse macrophages binding IgG3. J. Exp. Med. 153:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, R. D., J. E. May, M. A. Kane, M. M. Frank, and J. E. Bennett. 1974. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J. Immunol. 112:2260-2270. [PubMed] [Google Scholar]
- 8.Diamond, R. D., J. E. May, M. Kane, M. M. Frank, and J. E. Bennett. 1973. The role of late complement components and the alternate complement pathway in experimental cryptococcosis (37580). Proc. Soc. Exp. Biol. Med. 144:312-315. [DOI] [PubMed] [Google Scholar]
- 9.Dodds, A. W., and R. B. Sim. 1997. Complement: a practical approach. Oxford University Press, New York, N.Y.
- 10.Dong, Z. M., and J. W. Murphy. 1995. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect. Immun. 63:770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dromer, F., C. Perronne, J. Barge, J. L. Vilde, and P. Yeni. 1989. Role of IgG and complement component C5 in the initial course of experimental Cryptococcosis. Clin. Exp. Immunol. 78:412-417. [PMC free article] [PubMed] [Google Scholar]
- 12.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 14.Feldmesser, M., J. Mukherjee, and A. Casadevall. 1996. Combination of 5-flucytosine and capsule binding monoclonal antibody in therapy of murine Cryptococcus neoformans infections and in vitro. J. Antimicrob. Chemother. 37:617-622. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser, M., Y. Kress, and A. Casadevall. 1998. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J. Infect. Dis. 177:1639-1646. [DOI] [PubMed] [Google Scholar]
- 16.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnie, J. C., R. B. Stewart, and W. P. Aston. 1981. A comparison of cobra venom factor-induced depletion of serum C3 in eight different strains of mice. Dev. Comp. Immun. 5:697-701. [DOI] [PubMed] [Google Scholar]
- 18.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 19.Graybill, J. R., and J. Ahrens. 1981. Immunization and complement interaction in host defense against murine Cryptococcosis. J. Reticuloendothel. Soc. 30:347-357. [PubMed] [Google Scholar]
- 20.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, M. C. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 21.Kozel, T. R. 1996. Activation of the complement system by pathogenic fungi. Clin. Microbiol. Rev. 9:34-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozel, T. R. 1993. Opsonization and phagocytosis of Cryptococcus neoformans. Arch. Med. Res. 24:211-218. [PubMed] [Google Scholar]
- 23.Kozel, T. R., A. Tabuni, B. J. Young, and S. M. Levitz. 1996. Influence of opsonization conditions on C3 deposition and phagocyte binding of large- and small-capsule Cryptococcus neoformans cells. Infect. Immun. 64:2336-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel, T. R., and G. S. Pfrommer. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect. Immun. 52:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel, T. R., G. S. Pfrommer, A. S. Guerlain, B. A. Highison, and G. J. Highison. 1988. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev. Infect. Dis. 10(Suppl. 2):S436-S439. [DOI] [PubMed] [Google Scholar]
- 26.Kozel, T. R., M. A. Wilson, and J. W. Murphy. 1991. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect. Immun. 59:3101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel, T. R., M. A. Wilson, G. S. Pfrommer, and A. M. Schlageter. 1989. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect. Immun. 57:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozel, T. R., W. F. Gulley, and J. Cazin, Jr. 1977. Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect. Immun. 18:701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. Zuckier. 1998. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 177:1647-1659. [DOI] [PubMed] [Google Scholar]
- 30.Levitz, S. M. 1998. Host-fungal interactions in HIV infection. Res. Immunol. 149:489-493. [DOI] [PubMed] [Google Scholar]
- 31.Levitz, S. M. 1992. Overview of host defenses in fungal infections. Clin. Infect. Dis. 14(Suppl. 1):S37-S42. [DOI] [PubMed] [Google Scholar]
- 32.Levitz, S. M., A. Tabuni, T. R. Kozel, R. S. MacGill, D. T. Ingalls, and R. R. Golenbock. 1997. Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect. Immun. 65:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macher, A. M., J. E. Bennett, J. E. Gadek, and M. M. Frank. 1978. Complement depletion in cryptococcal sepsis. J. Immunol. 120:1686-1690. [PubMed] [Google Scholar]
- 34.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee, S., S. Lee, J. Mukherjee, M. D. Scharff, and A. Casadevall. 1994. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect. Immun. 62:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, J. W. 1998. Protective cell-mediated immunity against Cryptococcus neoformans. Res. Immunol. 149:373-386. [DOI] [PubMed] [Google Scholar]
- 37.Murphy, J. W., and G. C. Cozad. 1972. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect. Immun. 5:896-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum, G., R. Yuan, A. Casadevall, and M. D. Scharff. 1996. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J. Exp. Med. 183:1905-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nussbaum, G., W. Cleare, A. Casadevall, M. D. Scharff, and P. Valadon. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettoello-Mantovani, M., A. Casadevall, T. R. Kollmann, A. Rubinstein, and H. Goldstein. 1992. Enhancement of HIV-1 infection by the capsular polysaccharide of Cryptococcus neoformans. Lancet 339:21-23. [DOI] [PubMed] [Google Scholar]
- 41.Pietrella, D., C. Monari, C. Retini, B. Palazzettti, T. R. Kozel, and A. Vecchiarelli. 1998. Cryptococcus neoformans and Candida albicans regulate CD4 expression on human monocytes. J. Infect. Dis. 178:1464-1471. [DOI] [PubMed] [Google Scholar]
- 42.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 64:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes, J. C. 1985. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia 23:225-234. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes, J. C., L. S. Wicker, and W. Urba. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun. 29:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanford, J. E., D. M. Lupan, A. M. Schlageter, and T. R. Kozel. 1990. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect. Immun. 58:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundstrom, J. B., and R. Cherniak. 1993. T-cell dependent and T-cell independent mechanisms of tolerance to glucuronoxylomannan of Cryptococcus neoformans serotype A. Infect. Immun. 61:1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syme, R. M., T. F. Bruno, T. R. Kozel, and C. H. Mody. 1999. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 67:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 49.Vecchiarelli, A., C. Monari, C. Retini, D. Pietrella, B. Palazzetti, L. Pitzurra, and A. Casadevall. 1998. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86) expression on human monocytes. Eur. J. Immunol. 28:114-121. [DOI] [PubMed] [Google Scholar]
- 50.Vecchiarelli, A., C. Retini, C. Monari, and A. Casadevall. 1998. Specific antibody to Cryptococcus neoformans alters human leukocyte cytokine synthesis and promotes T-cell proliferation. Infect. Immun. 66:1244-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogel, C. W., R. Bredehorst, D. C. Fritzinger, T. Grunwald, P. Ziegelmuller, and M. A. Kock. 1996. Structure and function of cobra venom factor, the complement-activating protein in cobra venom. In B. R. Singh and A. T. Tu (ed.), Natural toxins II. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 52.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wetsel, R. A., S. T. Fleischer, and D. L. Haviland. 1990. Deficiency of the murine fifth complement component (C5). J. Biol. Chem. 265:2435-2440. [PubMed] [Google Scholar]
- 54.Wheat, W. H., R. Wetsel, A. Falus, B. F. Tack, and R. C. Strunk. 1987. The fifth component of complement (C5) in the mouse. J. Exp. Med. 165:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan, R. R., A. Casadevall, G. Spira, and M. D. Scharff. 1995. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J. Immunol. 154:1810-1816. [PubMed] [Google Scholar]
- 56.Yuan, R. R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan, R. R., G. Spira, J. Oh, M. Paizi, A. Casadevall, and M. D. Scharff. 1998. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect. Immun. 66:1057-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan, R. R., R. Clynes, J. Oh, J. V. Ravetch, and M. D. Scharff. 1998. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J. Exp. Med. 187:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuger, A., E. Louie, R. S. Holzman, M. S. Simberkoff, and J. J. Rahal. 1986. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann. Intern. Med. 104:234-240. [DOI] [PubMed] [Google Scholar]