Abstract
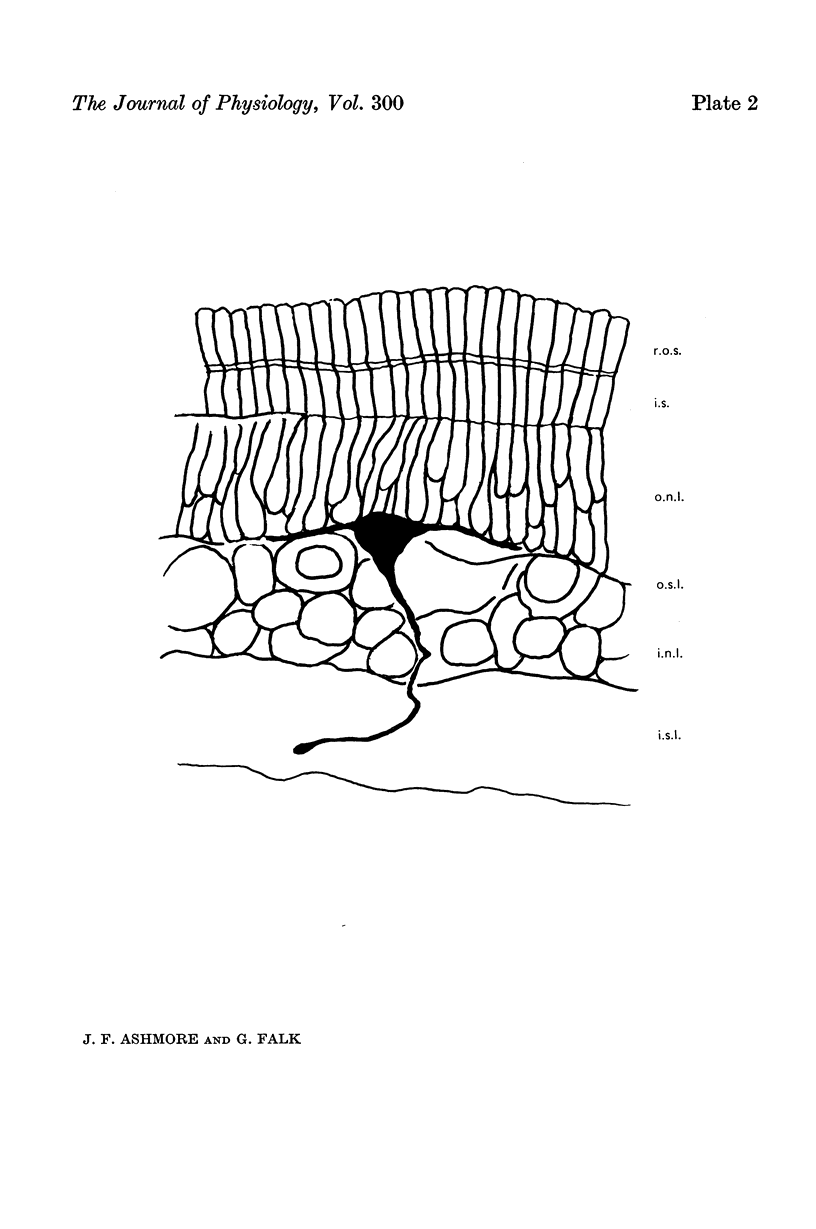
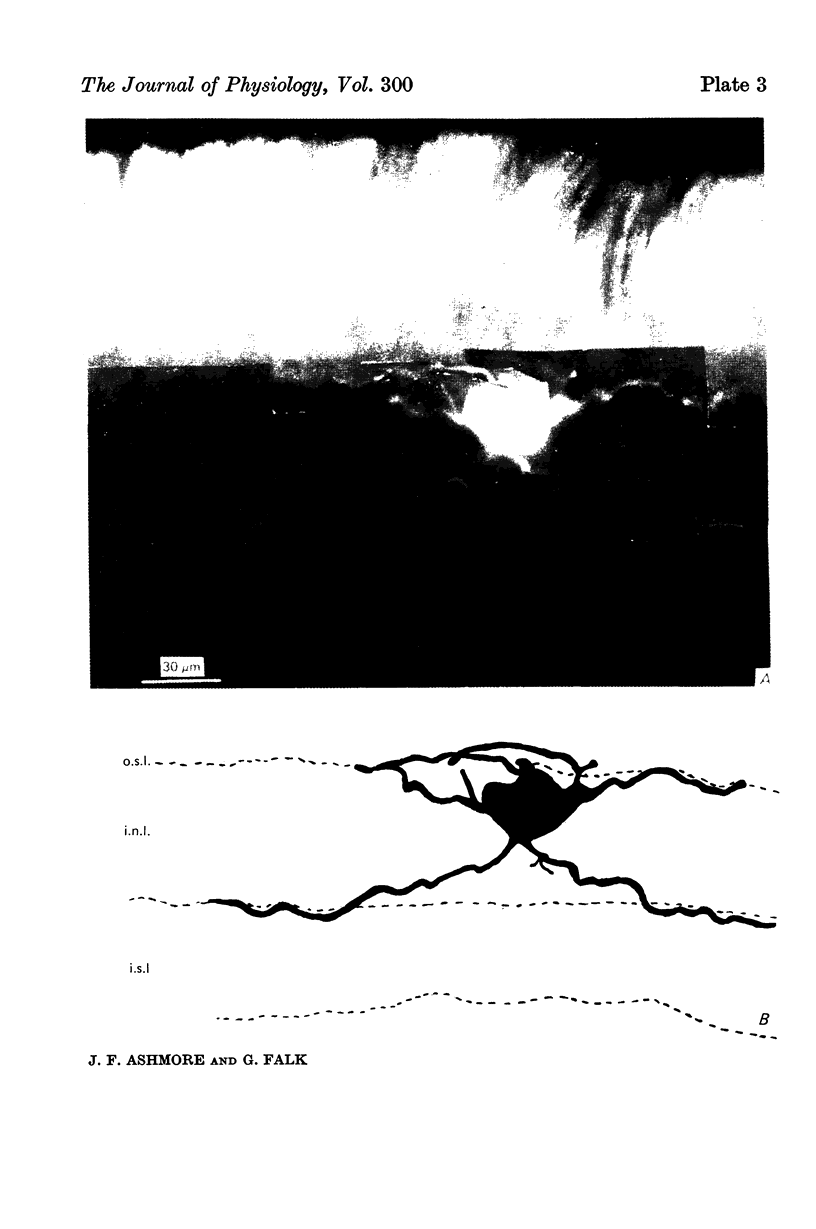
1. Responses to light were recorded from bipolar cells in the retina of the dogfish, Scyliorhinus canicula, under dark-adapted conditions. The identity of the cells was confirmed by Procion Yellow staining.
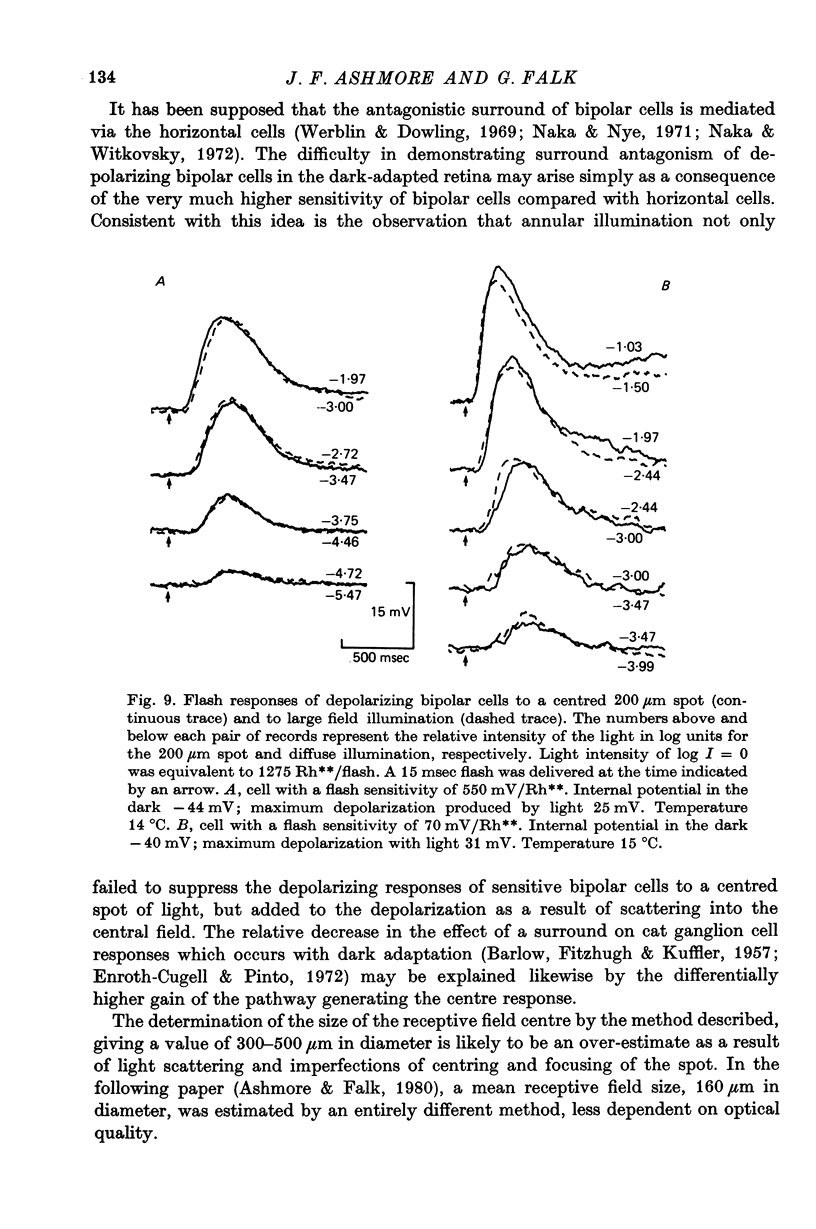
2. More than 95% of the bipolar cells sampled were of the type which depolarized to a spot of light. These are termed depolarizing bipolar cells. In most cells, illumination of the surround had little effect on the responses elicited from the central receptive field.
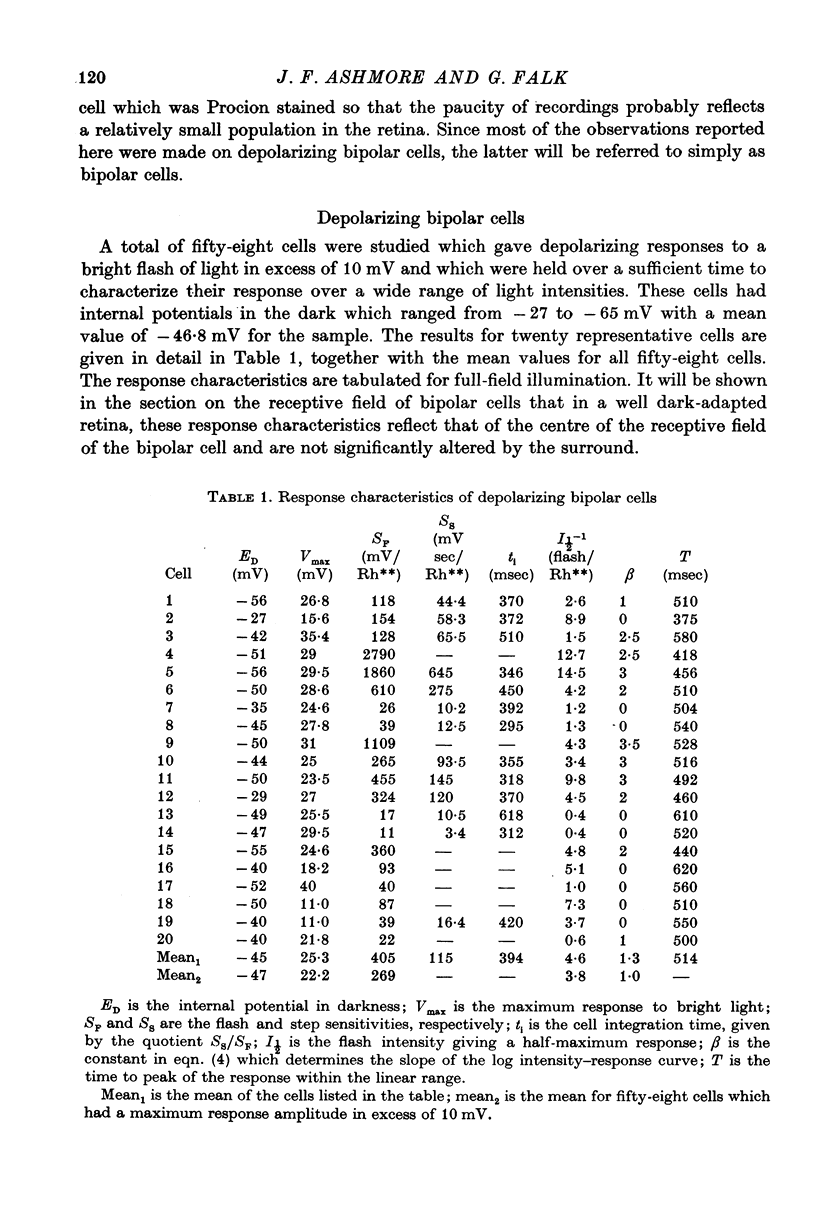
3. The mean flash sensitivity of the depolarizing bipolar cells was 270 mV/Rh** (where Rh** signifies rhodopsin photoisomerization per rod for full field illumination).
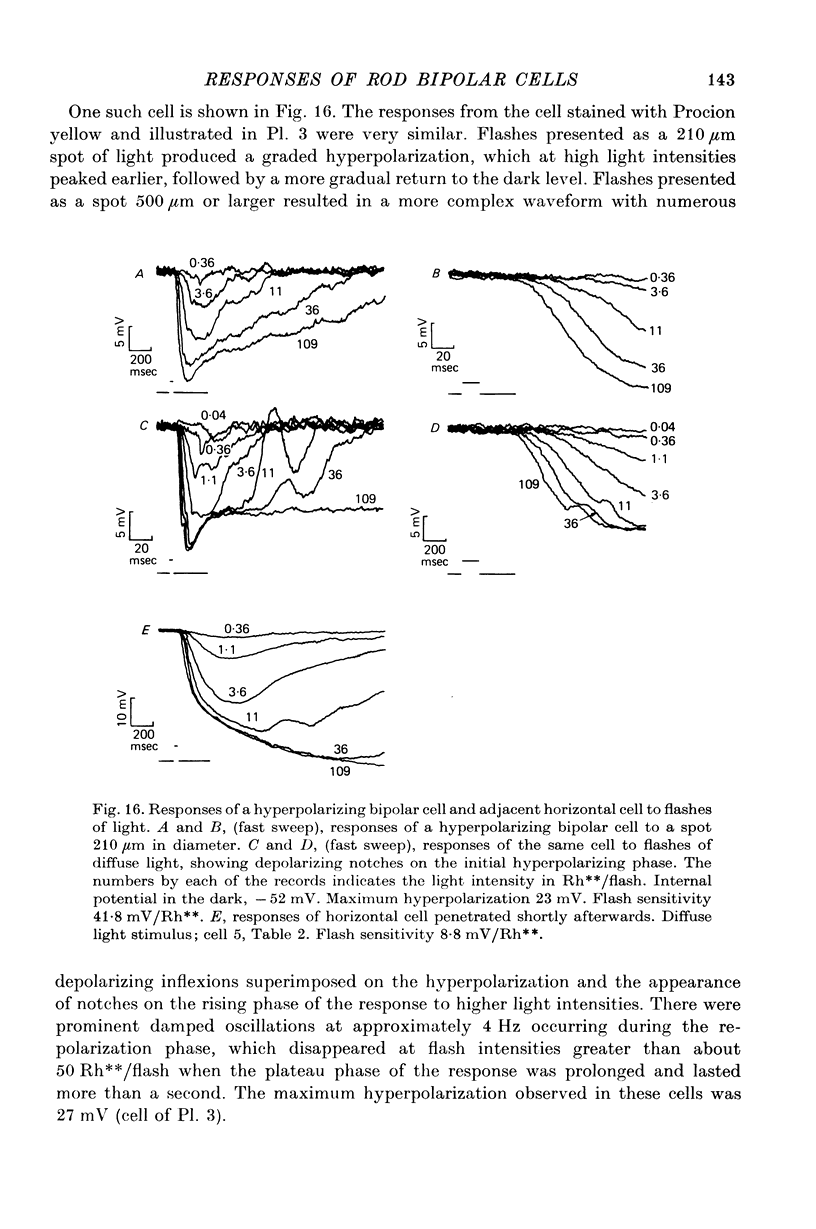
4. The mean flash sensitivity of horizontal cells under the same conditions was 8 mV/Rh**. In a limited sample of hyperpolarizing bipolar cells the highest flash sensitivity was 42 mV/Rh**.
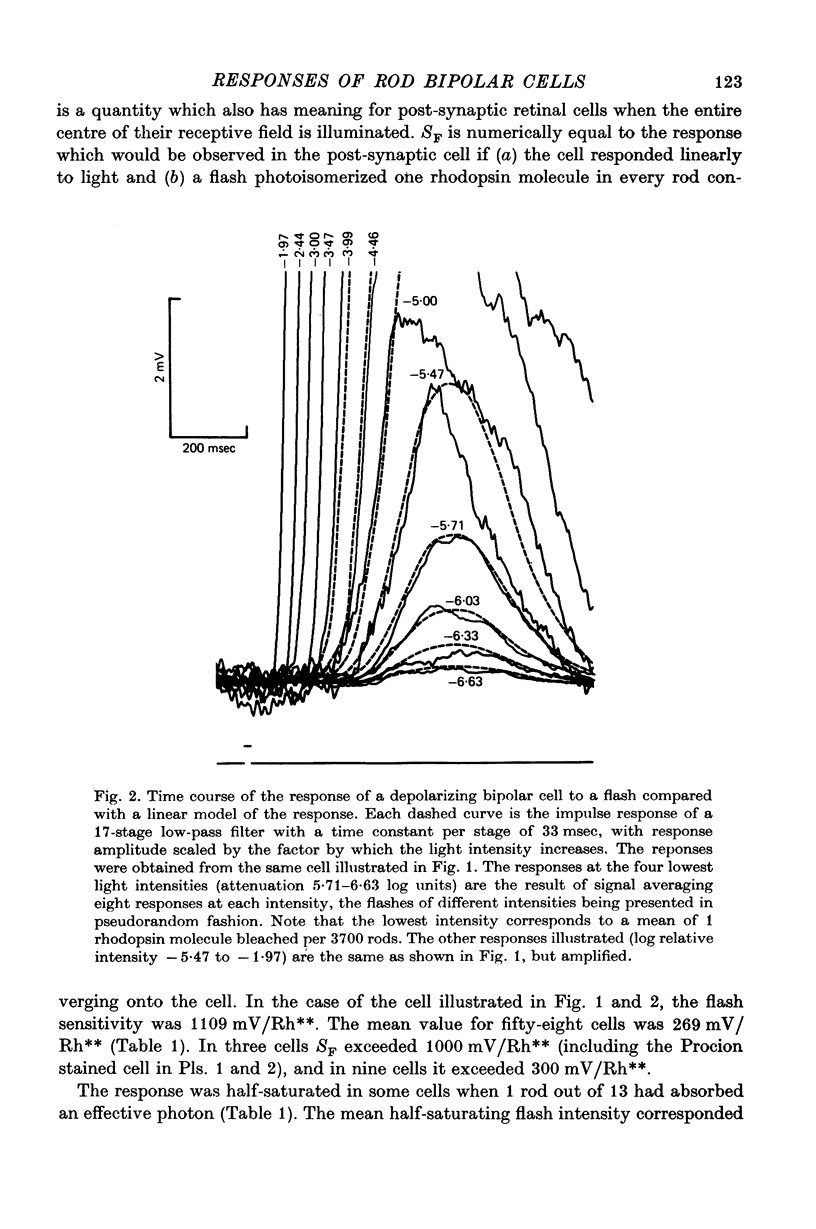
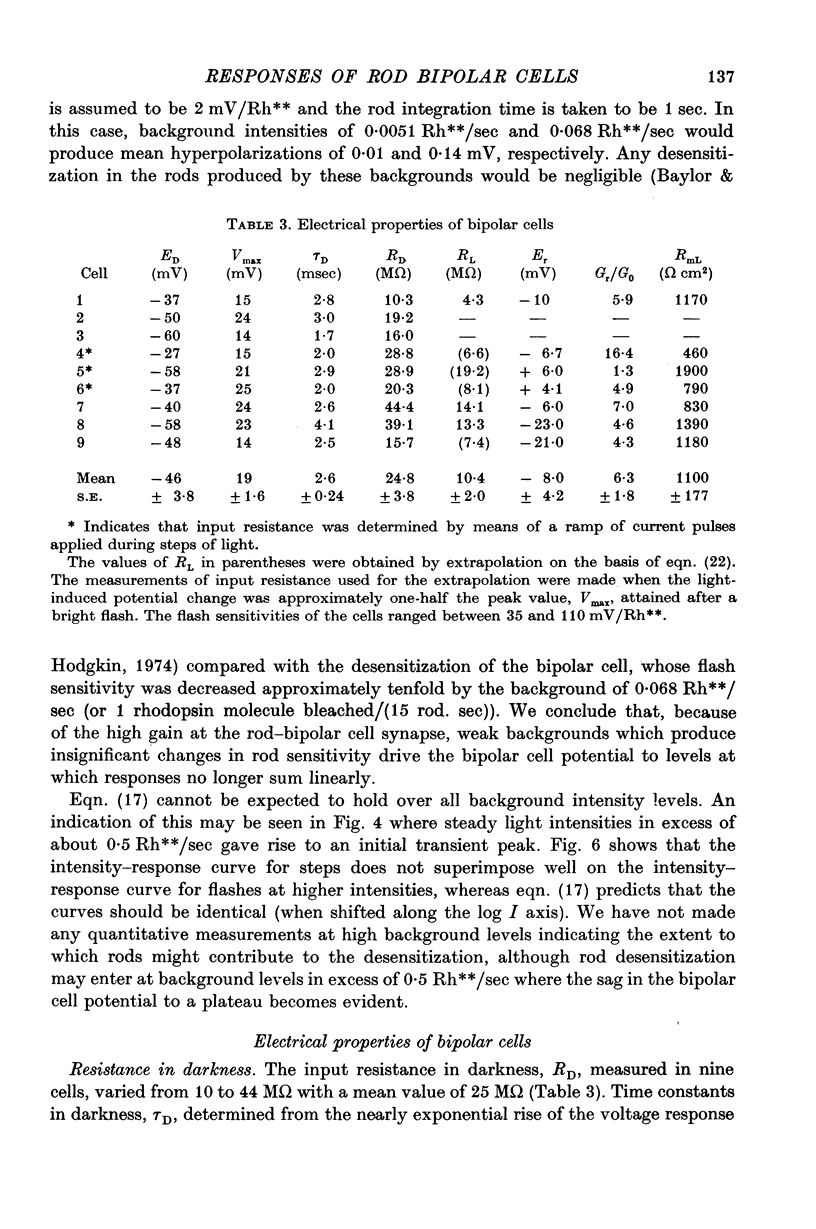
5. The high flash sensitivity of the depolarizing bipolar cells indicates a large voltage gain at its synapse with rods. On the assumption of a rod flash sensitivity of 2 mV/Rh** the mean gain at the synapse was 135, but for some cells the gain was in excess of 500.
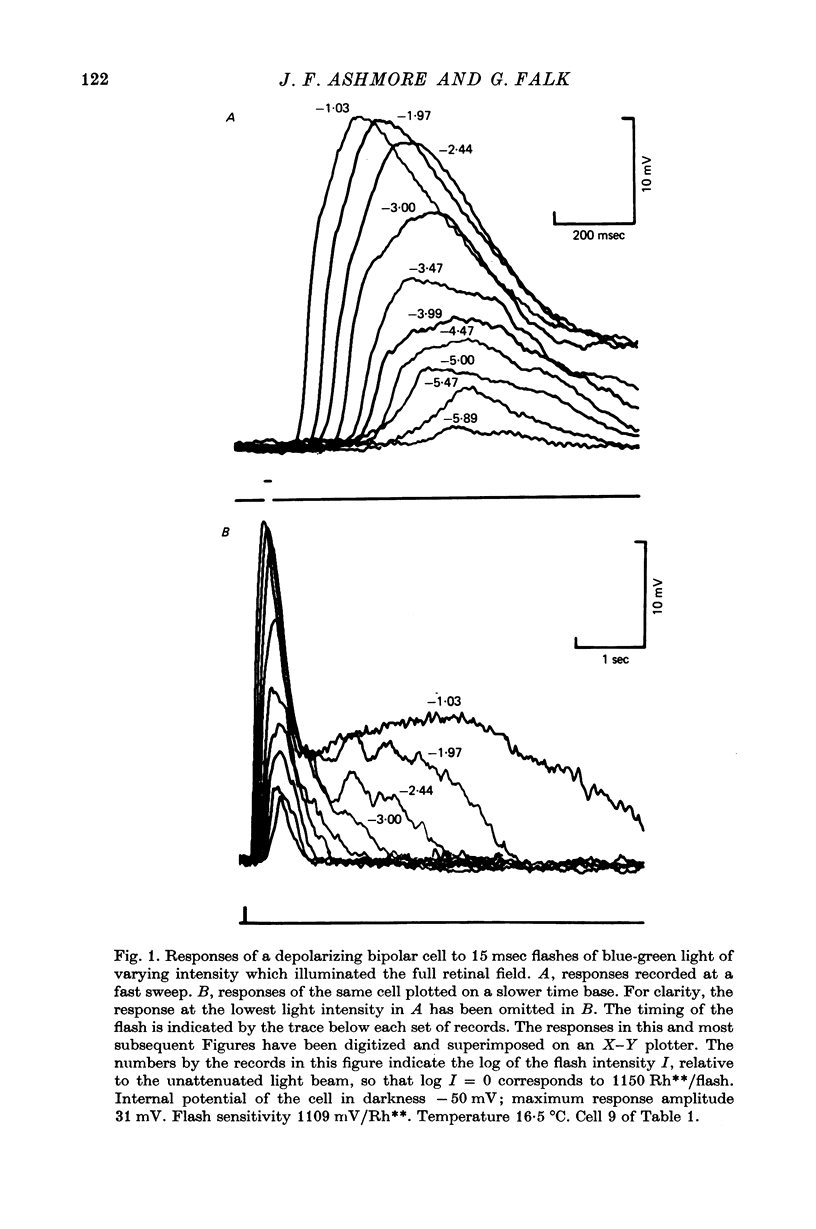
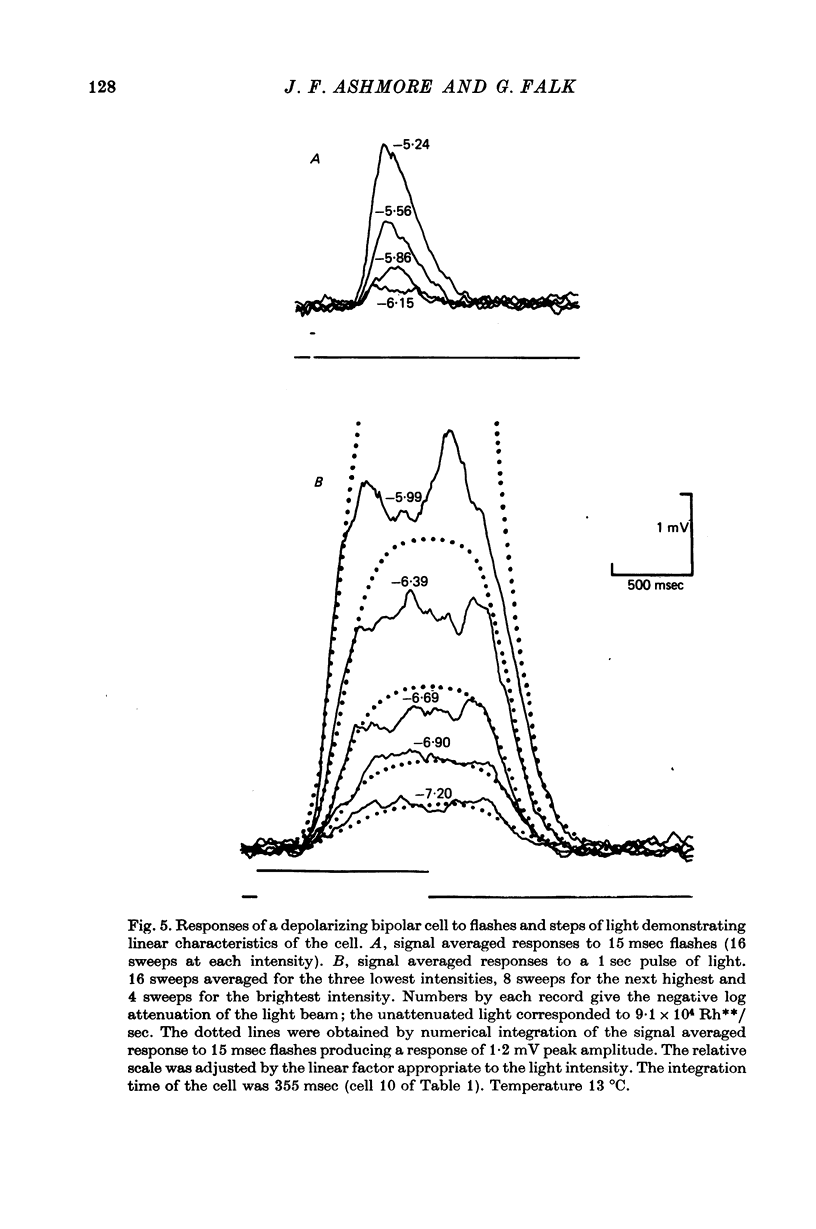
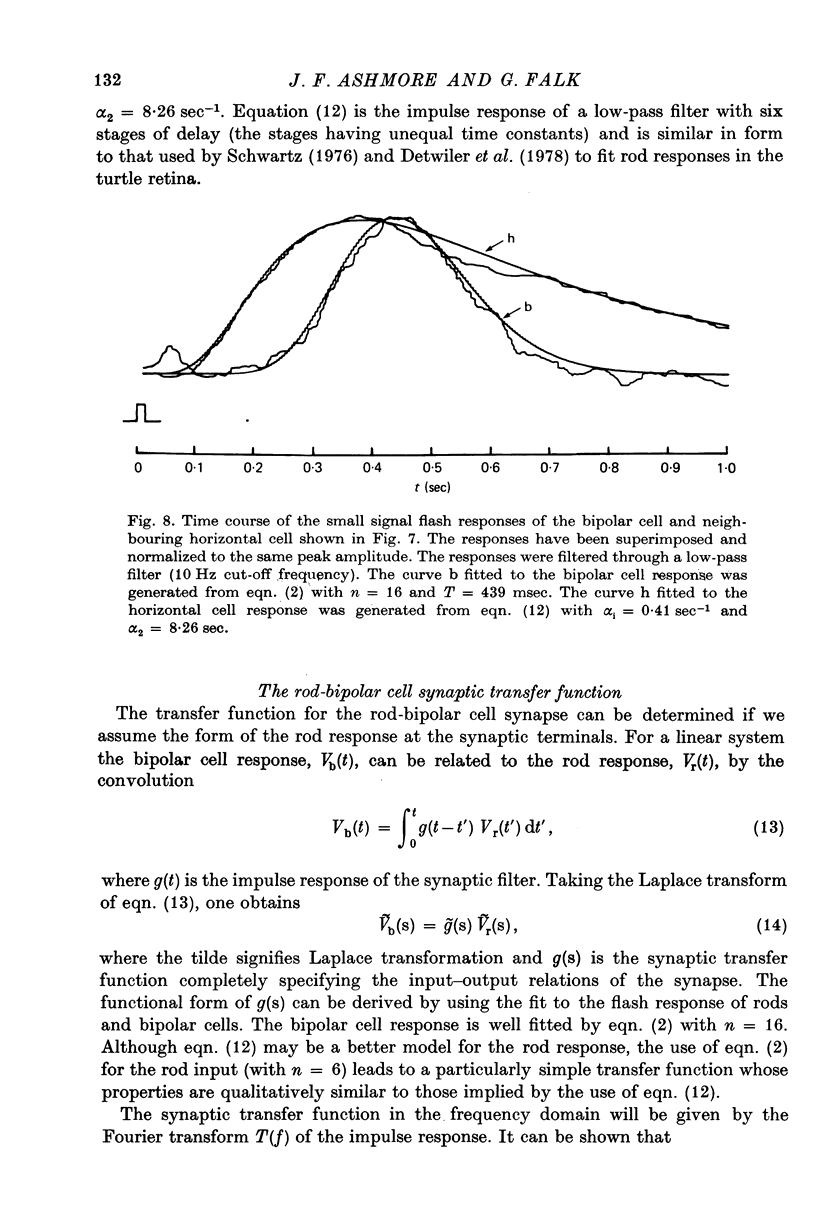
6. Responses of depolarizing bipolar cells to dim flashes could be approximated by the impulse response of a 12-16 stage low-pass filter, whereas horizontal cell responses could be fitted by a low-pass filter of six sections. The implied filter at the rod-bipolar cell synapse is tuned to the higher frequency components of rod signals, thereby improving temporal resolution in the rod pathway.
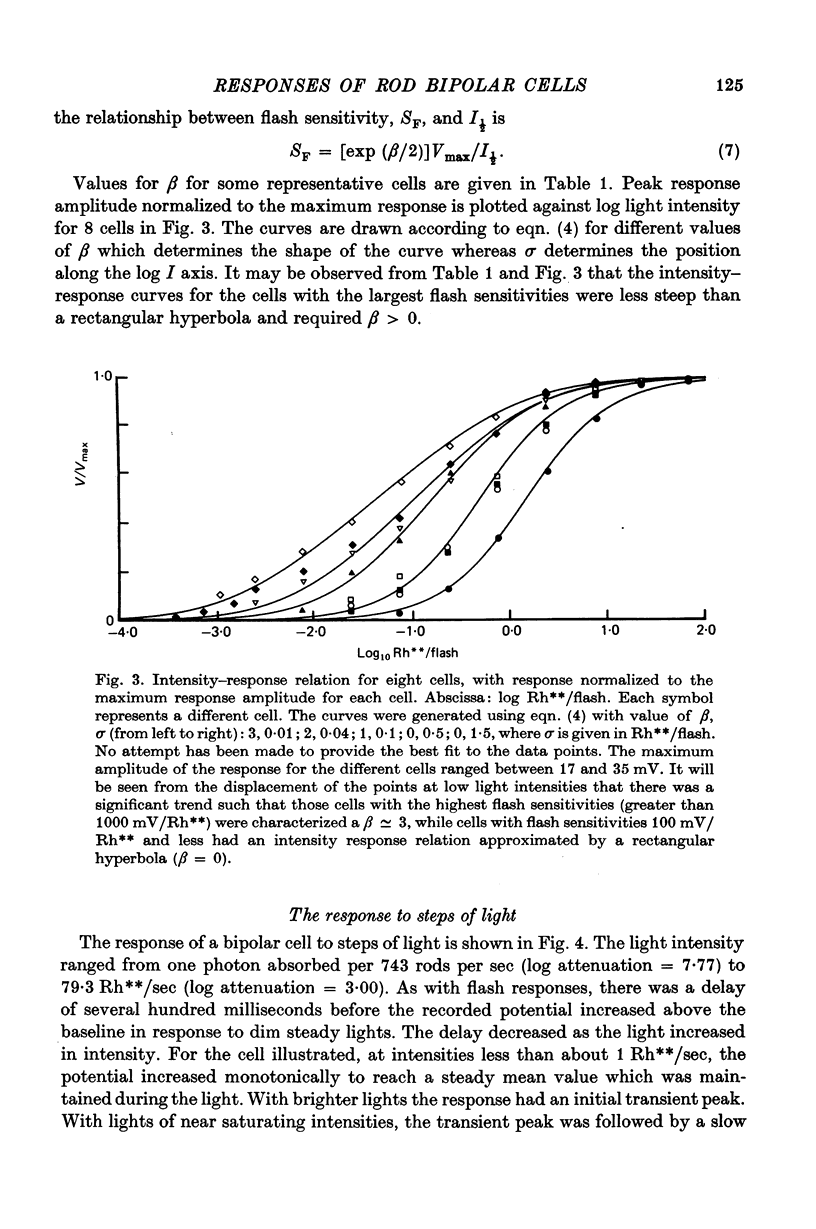
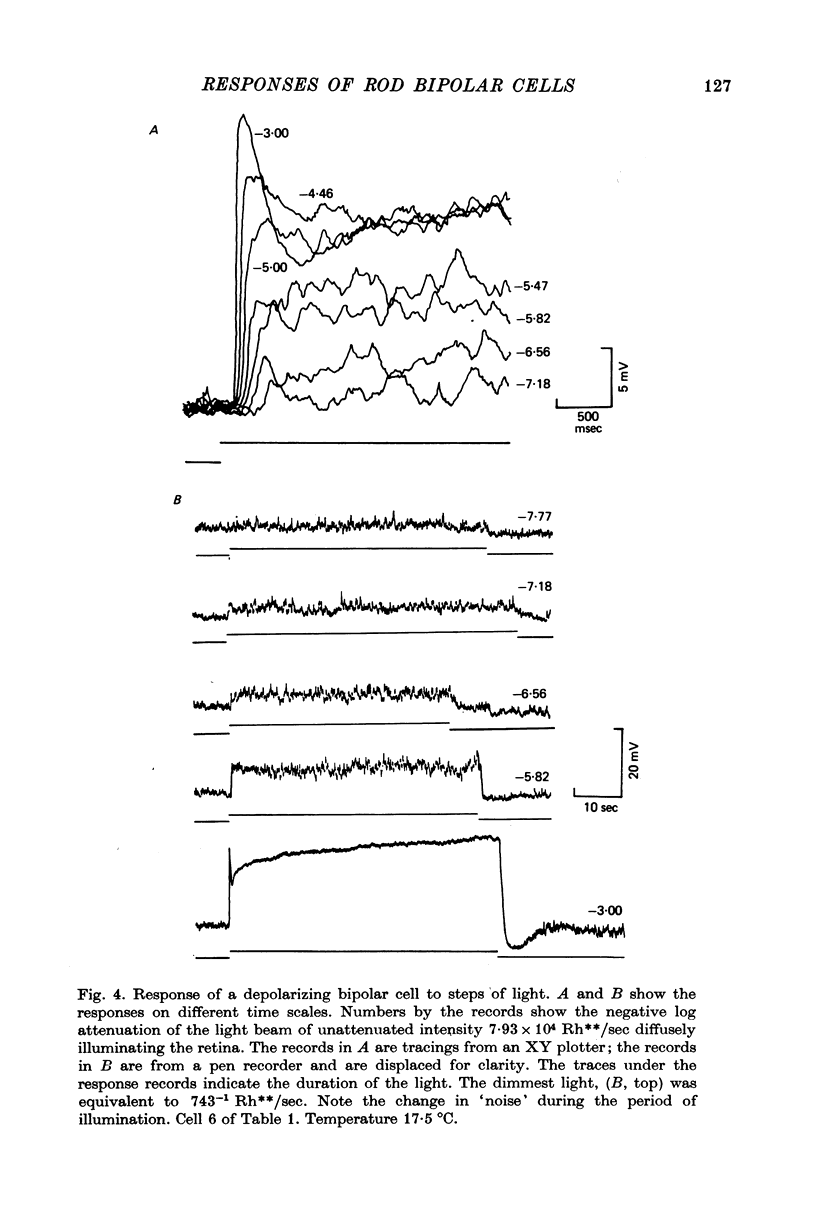
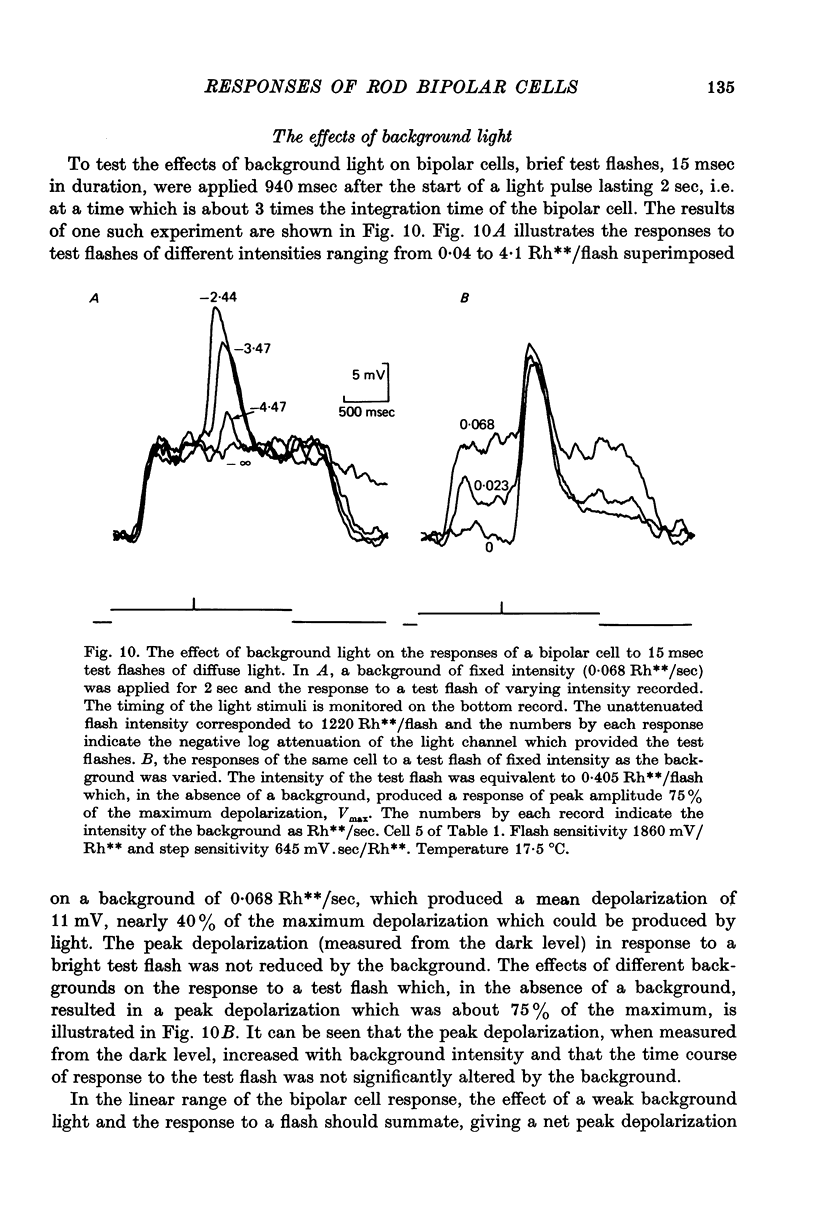
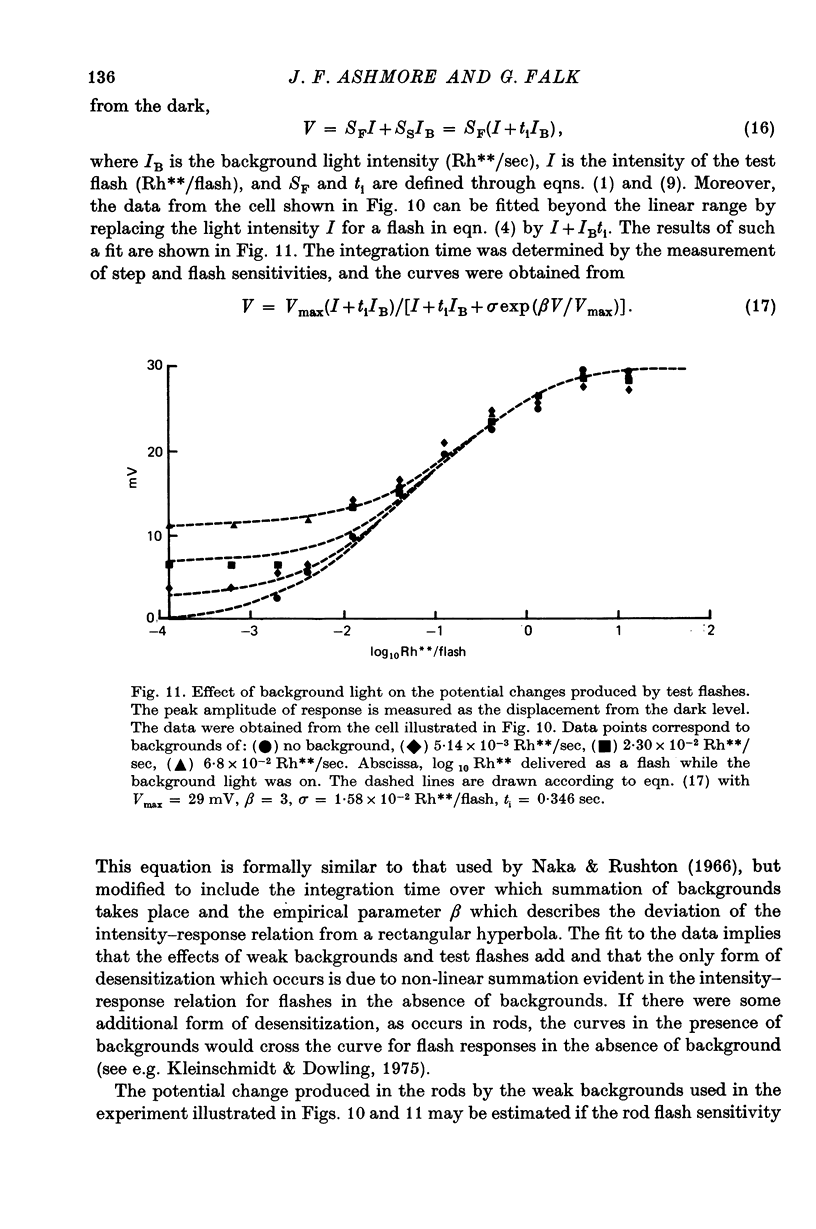
7. Depolarizing bipolar cell responses to test flashes are reduced by weak background illumination (less than 0·1 Rh**/sec). This desensitization, which would not be expected to affect rod responses, could be explained by a shift in the operating point to a less sensitive region of the intensity—response curve as a result of the large depolarization elicited by the background.
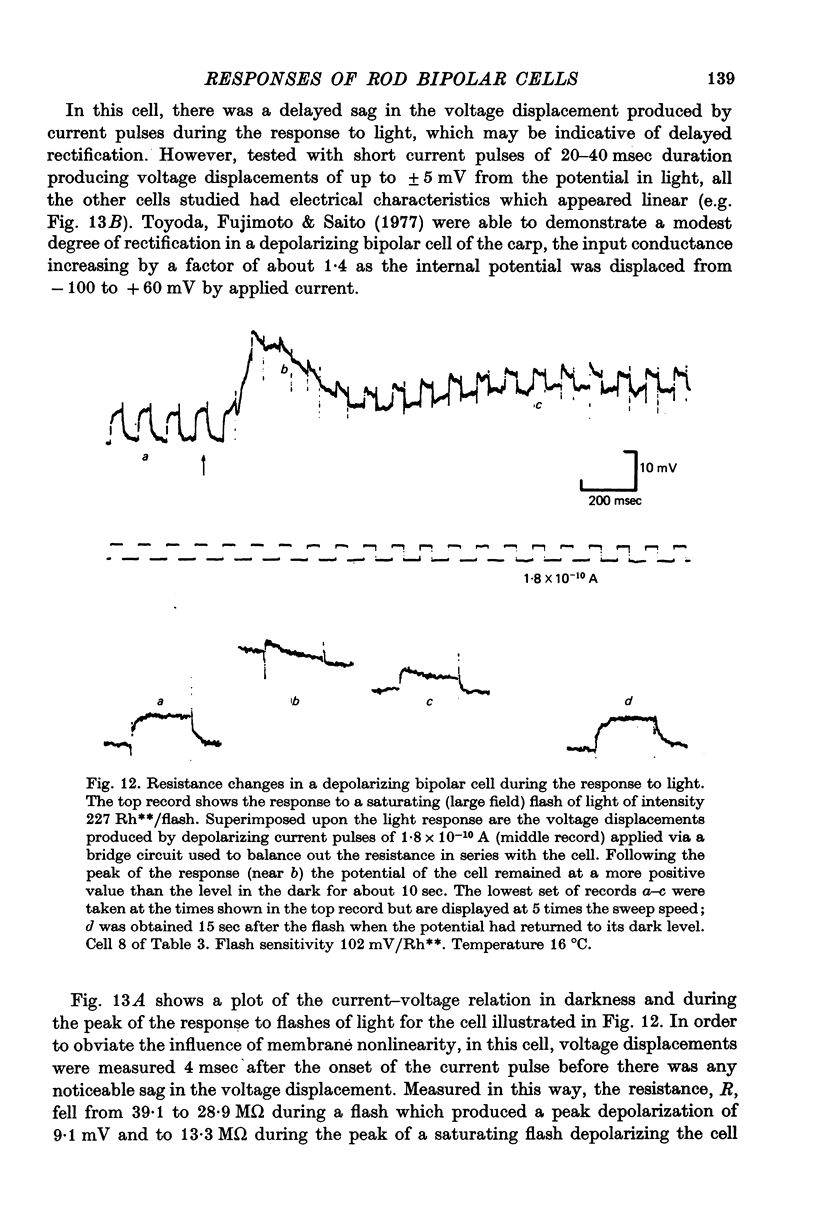
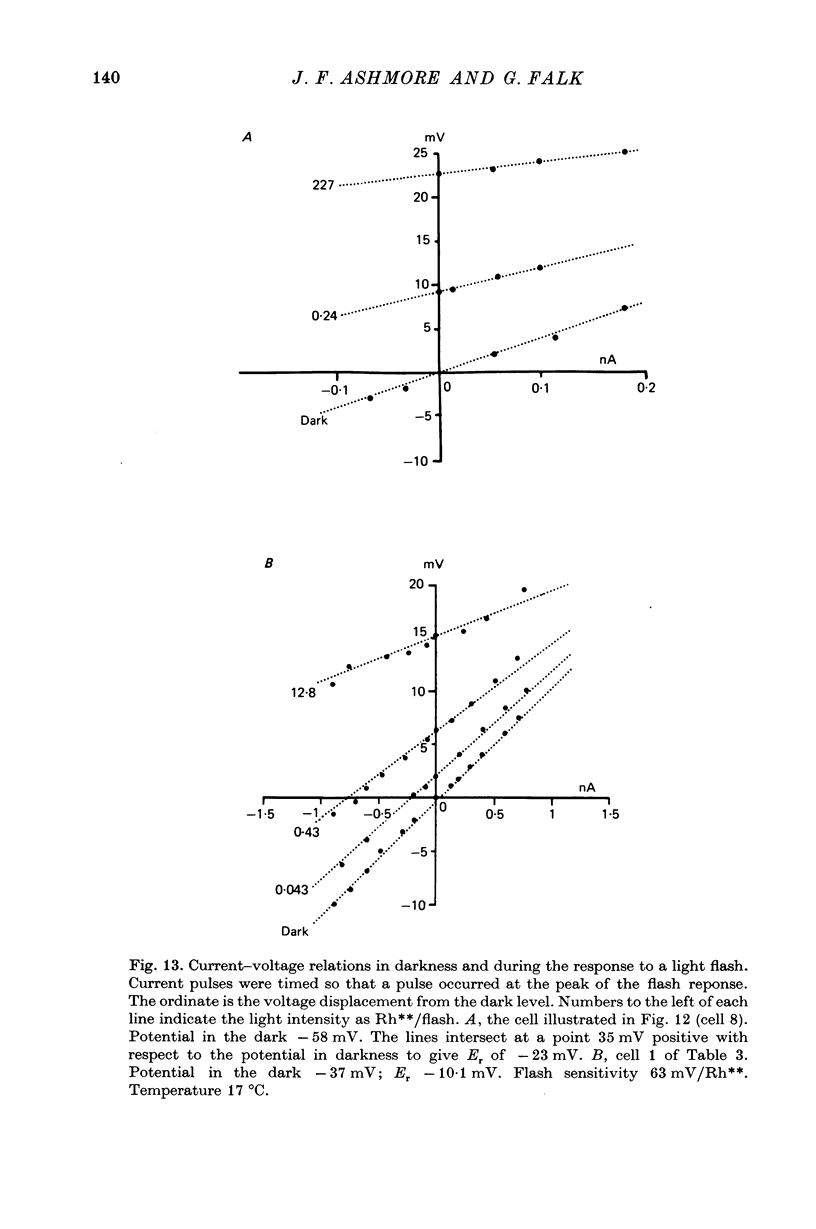
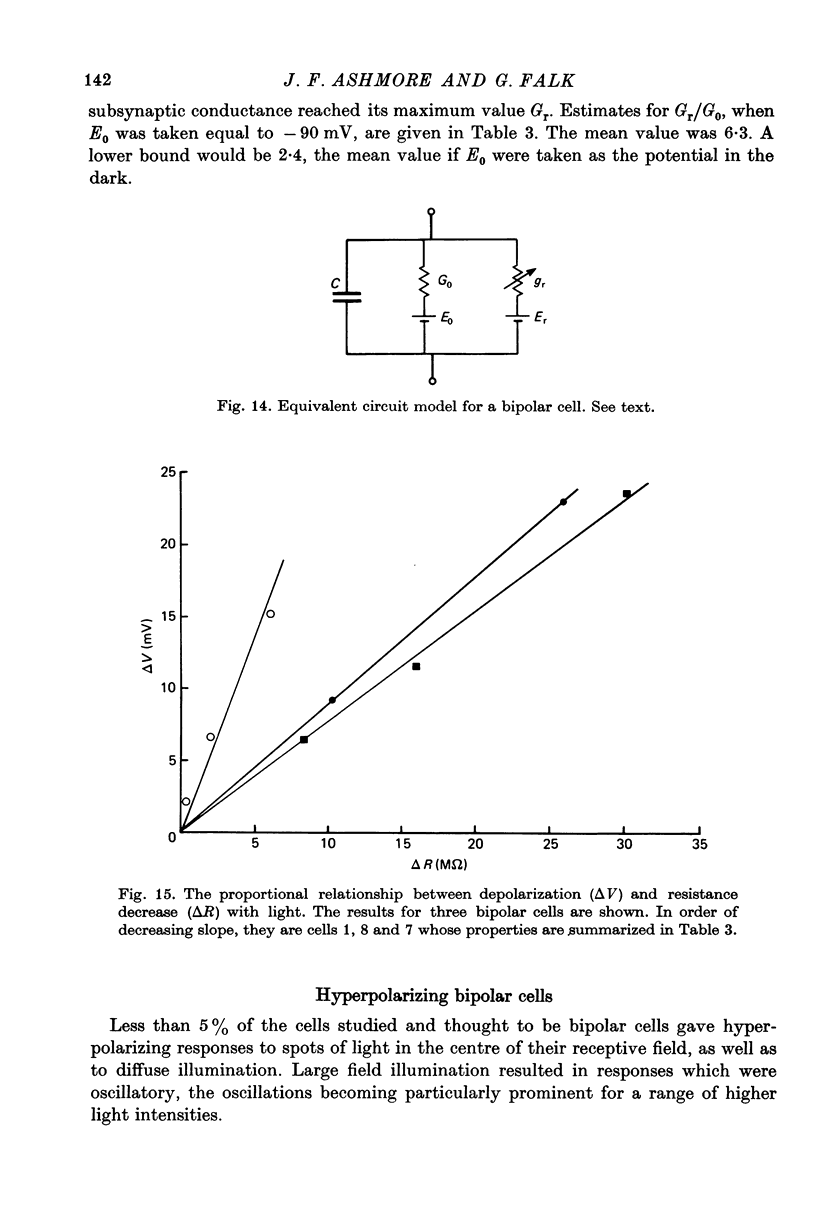
8. The results of current injection into the cell in darkness and during the response to light are consistent with the release by rod terminals of a transmitter which closes ionic channels in a conductance path having a reversal potential of - 8 mV, transmitter release being suppressed by light.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Proceedings: Some properties of bipolar cells in the retina of dogfish and rays. J Physiol. 1976 Jun;258(2):39P–40P. [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. The single-photon signal in rod bipolar cells of the dogfish retina. J Physiol. 1980 Mar;300:151–166. doi: 10.1113/jphysiol.1980.sp013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Transmission of visual signals to bipolar cells near absolute threshold. Vision Res. 1979;19(4):419–423. doi: 10.1016/0042-6989(79)90107-x. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S., Baker R. F. LONGITUDINAL IMPEDANCE OF THE SQUID GIANT AXON. J Gen Physiol. 1941 Jul 20;24(6):771–788. doi: 10.1085/jgp.24.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Yamane S. Effects of adapting lights on the time course of the receptor potential of the anuran retinal rod. J Physiol. 1975 May;247(1):189–207. doi: 10.1113/jphysiol.1975.sp010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. A surprising property of electrical spread in the network of rods in the turtle's retina. Nature. 1978 Aug 10;274(5671):562–565. doi: 10.1038/274562a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. Letter: The dynamic voltage-transfer function for rod-bipolar cell transmission. Vision Res. 1974 Aug;14(8):739–741. doi: 10.1016/0042-6989(74)90073-x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden W. L., Jr, Dowling J. E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological studies of single retinal cells and their morphological identification. Vision Res. 1971;Suppl 3:17–26. doi: 10.1016/0042-6989(71)90027-7. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootsey J. M., Johnson E. A. Buffer amplifier with femtofarad input capacity using operational amplifiers. IEEE Trans Biomed Eng. 1973 Sep;20(5):389–391. doi: 10.1109/TBME.1973.324240. [DOI] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L., Torre V. The responses of amacrine cells to light and intracellularly applied currents. J Physiol. 1978 Mar;276:83–102. doi: 10.1113/jphysiol.1978.sp012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Ringham G. L. Synaptic transfer at a vertebrate central nervous system synapse. J Physiol. 1975 Oct;251(2):409–426. doi: 10.1113/jphysiol.1975.sp011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Nye P. W. Role of horizontal cells in organization of the catfish retinal receptive field. J Neurophysiol. 1971 Sep;34(5):785–801. doi: 10.1152/jn.1971.34.5.785. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Witkovsky P. Dogfish ganglion cell discharge resulting from extrinsic polarization of the horizontal cells. J Physiol. 1972 Jun;223(2):449–460. doi: 10.1113/jphysiol.1972.sp009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Pasino E., Marchiafava P. L. Transfer properties of rod and cone cells in the retina of the tiger salamander. Vision Res. 1976;16(4):381–386. doi: 10.1016/0042-6989(76)90200-5. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. A theoretical treatment of Fuortes's observations upon eccentric cell activity in Limulus. J Physiol. 1959 Oct;148:29–38. doi: 10.1113/jphysiol.1959.sp006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Increment threshold and dark adaptation. J Opt Soc Am. 1963 Jan;53:104–109. doi: 10.1364/josa.53.000104. [DOI] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kondo H., Toyoda J. Rod and cone signals in the on-center bipolar cell: their different ionic mechanisms. Vision Res. 1978;18(5):591–595. doi: 10.1016/0042-6989(78)90208-0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K., Witkovsky P. Retinal structure in the smooth dogfish, Mustelus canis: general description and light microscopy of giant ganglion cells. J Comp Neurol. 1973 Mar 1;148(1):1–31. doi: 10.1002/cne.901480102. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Witkovsky P. Retinal structure in the smooth dogfish, Mustelus canis: light microscopy of photoreceptor and horizontal cells. J Comp Neurol. 1973 Mar 1;148(1):33–45. doi: 10.1002/cne.901480103. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Stell W. K. Retinal structure in the smooth dogfish Mustelus canis: electron microscopy of serially sectioned bipolar cell synaptic terminals. J Comp Neurol. 1973 Jul 15;150(2):147–167. doi: 10.1002/cne.901500204. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Stell W. K. Retinal structure in the smooth dogfish, Mustelus canis: light microscopy of bipolar cells. J Comp Neurol. 1973 Mar 1;148(1):47–59. doi: 10.1002/cne.901480104. [DOI] [PubMed] [Google Scholar]