Abstract
1. Impulses were recorded from single afferent fibres in the median and ulnar nerves of human subjects. The response of slowly adapting mechanosensitive units with receptive fields in the glabrous skin of the hand were studied when rectangular indentations of varying amplitudes and invariant time duration were delivered. Simultaneously the subject was asked to estimate the magnitude of his sensation associated with the stimuli.
2. Stimulus—response plots of the afferent units were constructed and compared with the psychophysical magnitude estimation plots.
3. The stimulus—response data of the afferent units fell along monotonous curves which were largely decelerating when stimuli above the static threshold were taken into account. When responses below the static threshold were taken into account many plots were S-shaped.
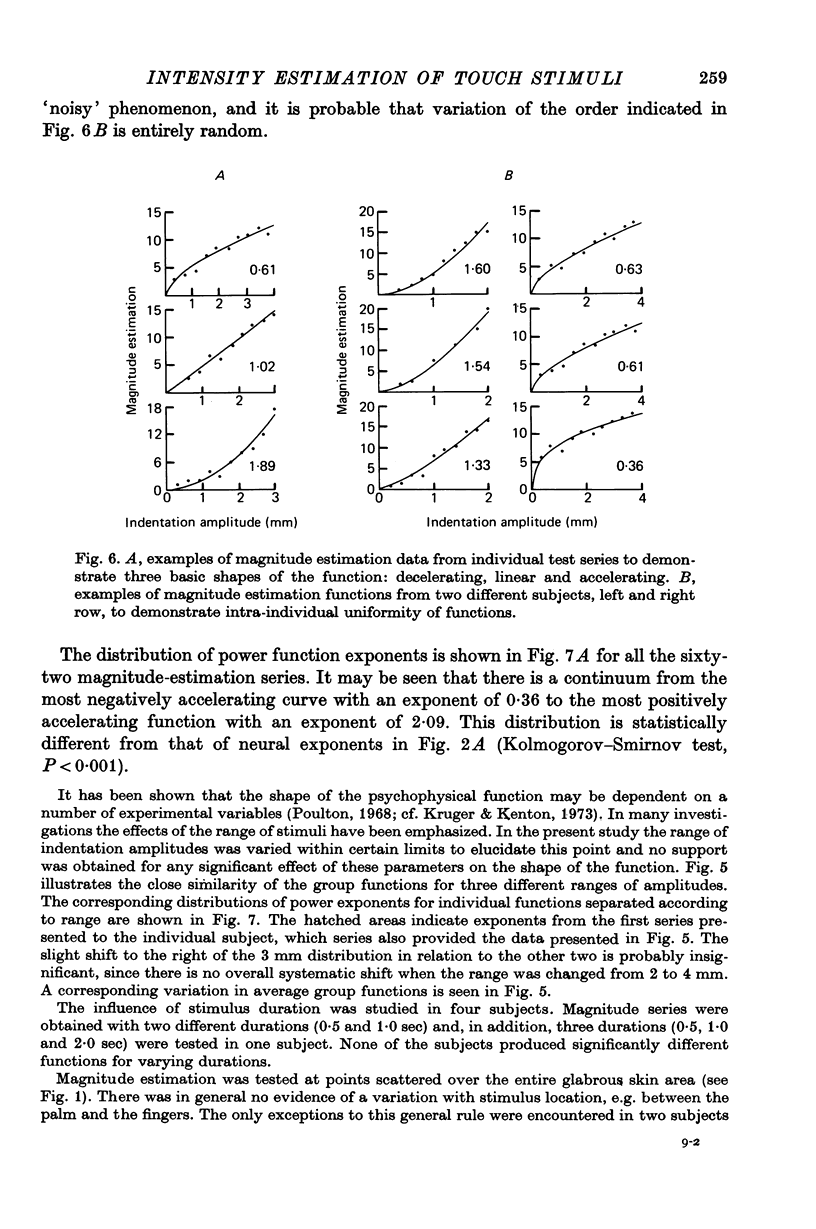
4. The psychophysical plots were monotonous and either decelerating, linear or accelerating.
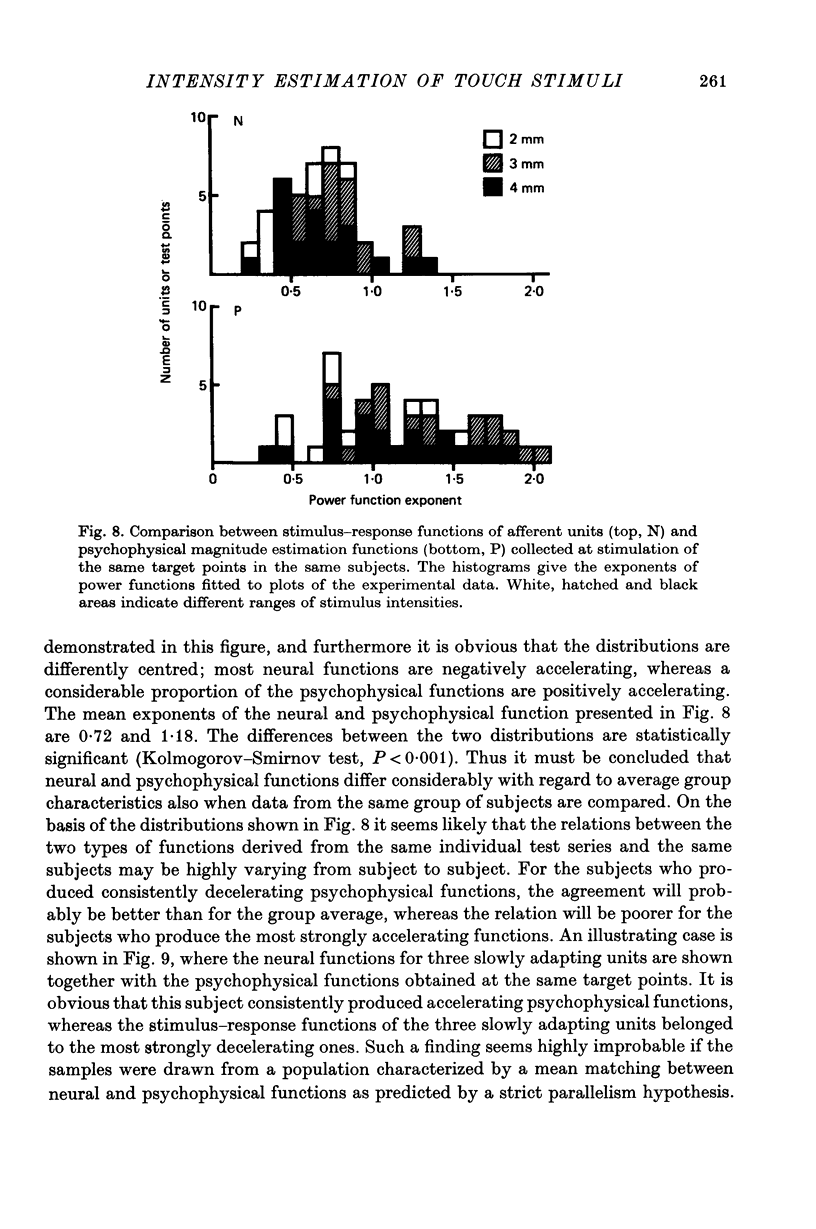
5. Power functions were fitted to the two sets of data. The group average differed considerably with regard to the exponent of the fitted functions which was 0·7 for the neural and 1·0 for the psychophysical function.
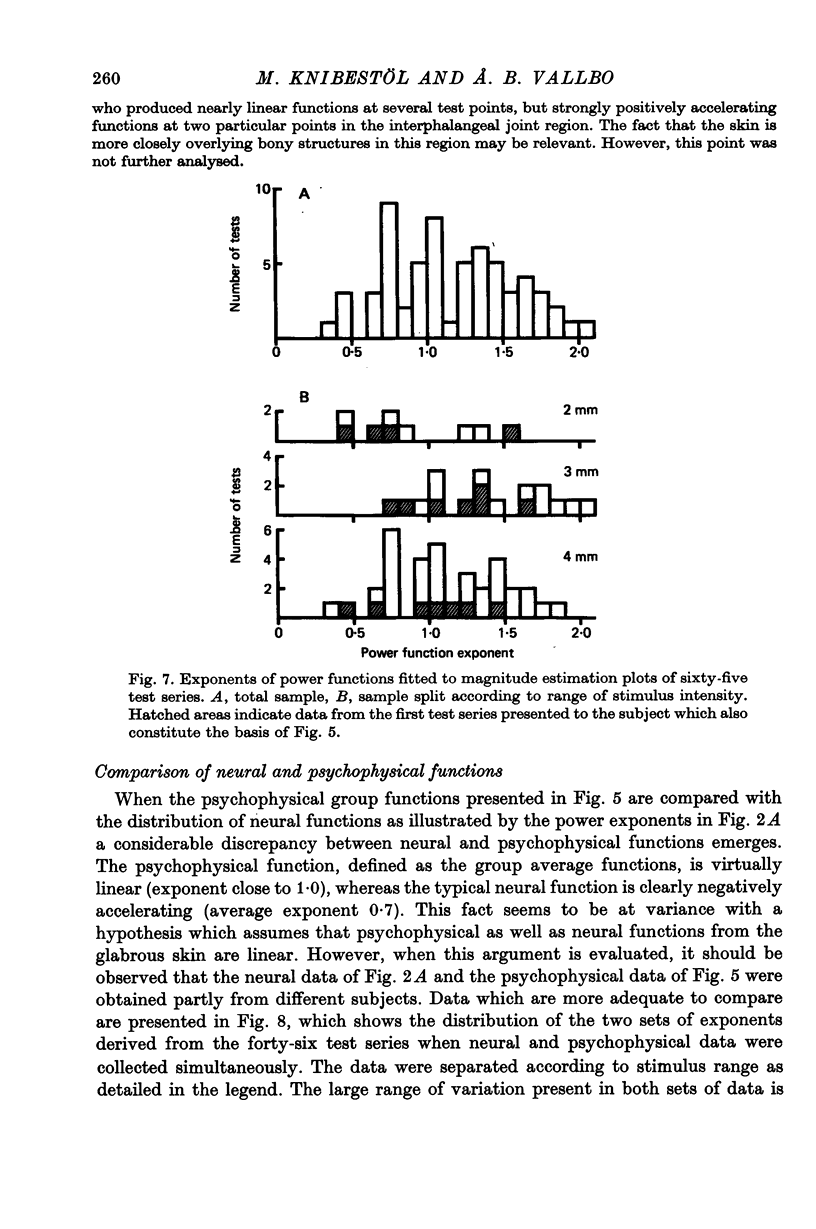
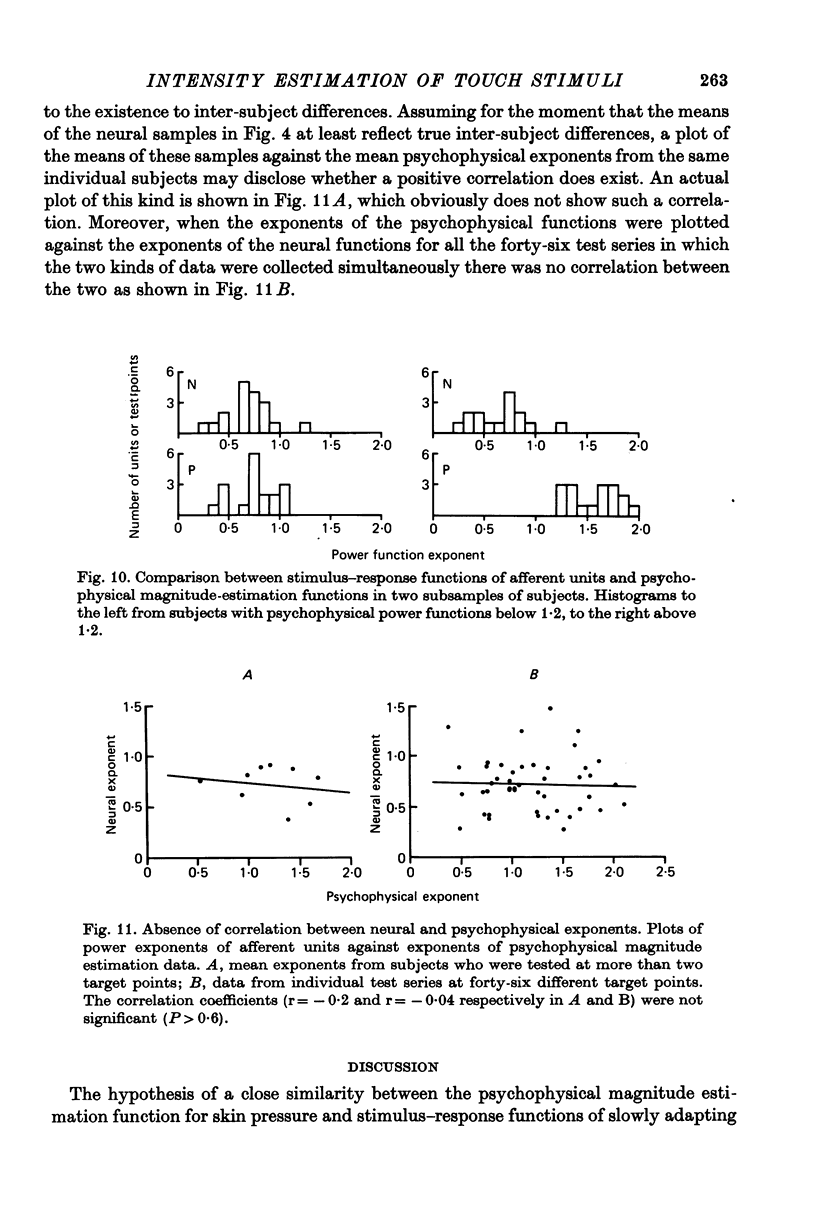
6. There was considerable variation between shapes of curves derived from individual test points. The range of exponents for the neural function was 0·26-1·92 and for the psychophysical function 0·36-2·09. The variation in psychophysical functions was partly accounted for by relatively stable inter-subject differences, whereas no such inter-subject difference was evident for the neural functions, which seemed to vary randomly.
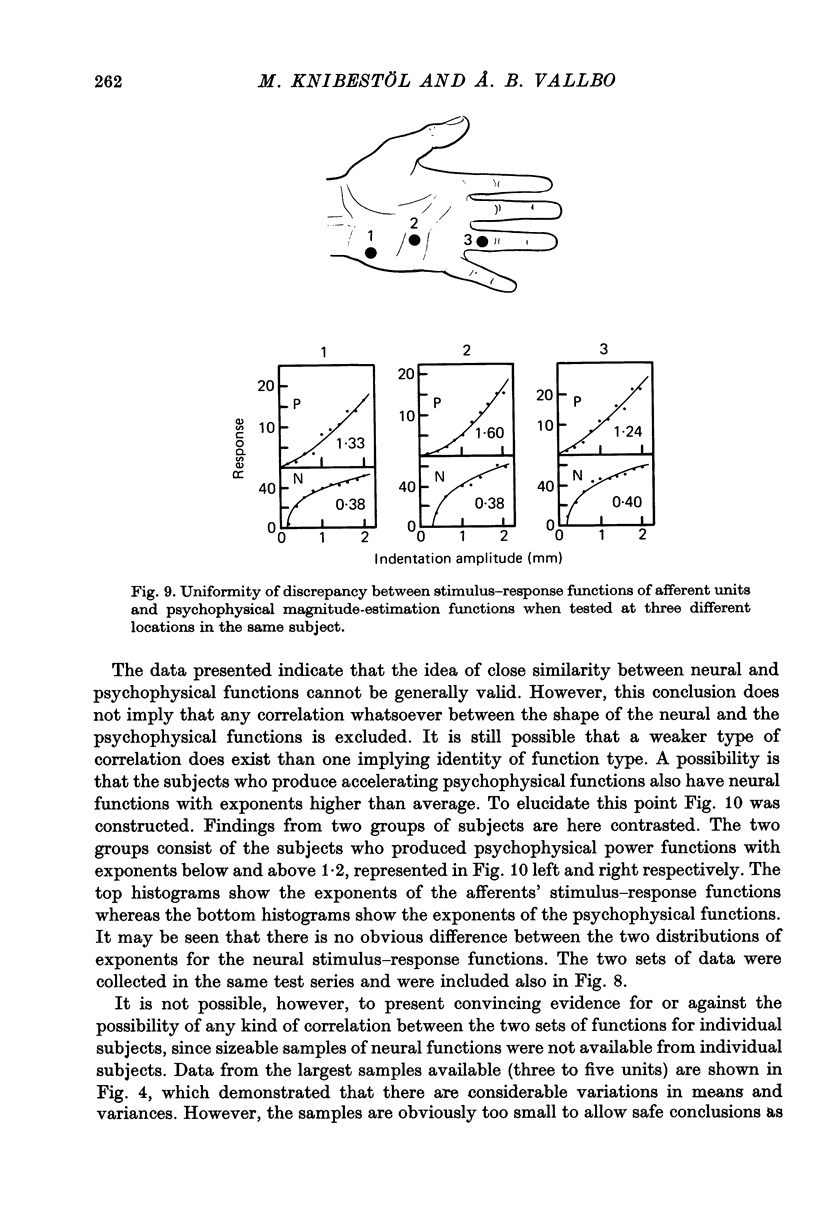
7. There was no indication of a correlation between the shapes of the neural functions and the shapes of the psychophysical functions when data from individual subjects or individual test points were compared. Moreover, when two groups of data were considered, one with accelerating and one with decelerating psychophysical functions, the associated neural functions did not differ between the two groups.
8. It was concluded that the hypothesis of a close agreement between the stimulus—response functions of slowly adapting mechanoreceptors in the human hand and the psychophysical magnitude estimation functions is not tenable. This was evident when average group data were compared as well as when data from individual subjects and individual target points were compared. The findings suggest that the shapes of the psychophysical magnitude estimation functions are highly dependent on central mechanisms and are not a direct function of the properties of the afferent units as has been claimed in previous investigations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Harrington T., Merzenich M. M. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10(3):251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol. 1978 Aug;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S., Vallbo A. B. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979 Jan;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. J Physiol. 1973 Aug;232(3):427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol. 1975 Feb;245(1):63–80. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M., Vallbo A. B. Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand. 1970 Oct;80(2):178–195. doi: 10.1111/j.1748-1716.1970.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Kruger L., Kenton B. Quantitative neural and psychophysical data for cutaneous mechanoreceptor function. Brain Res. 1973 Jan 15;49(1):1–24. doi: 10.1016/0006-8993(73)90398-3. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POGGIO G. F., WERNER G. THE RELATION OF THALAMIC CELL RESPONSE TO PERIPHERAL STIMULI VARIED OVER AN INTENSIVE CONTINUUM. J Neurophysiol. 1963 Sep;26:807–834. doi: 10.1152/jn.1963.26.5.807. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. The response of a single end organ. J Physiol. 1931 Jan 21;71(1):64–110. doi: 10.1113/jphysiol.1931.sp002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS J. C., MACK J. D. Scales of apparent force. J Exp Psychol. 1959 Nov;58:405–413. doi: 10.1037/h0046906. [DOI] [PubMed] [Google Scholar]
- STEVENS S. S. On the psychophysical law. Psychol Rev. 1957 May;64(3):153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- Stevens S. S. Intensity functions in sensory systems. Int J Neurol. 1967;6(2):202–209. [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968 Jul;21(3):270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]


