Abstract
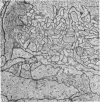
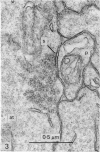
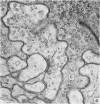
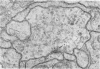
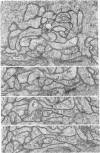
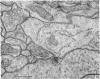
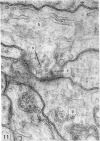
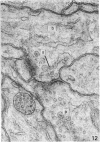
1. The contacts of horizontal cell dendrites with processes of other second order neurones were studied at the level of the electron microscope in serial sections of the salamander retina. Intracellular recordings of the responses to light of horizontal and bipolar cells were used to investigate the possible significance of some of the morphological findings.
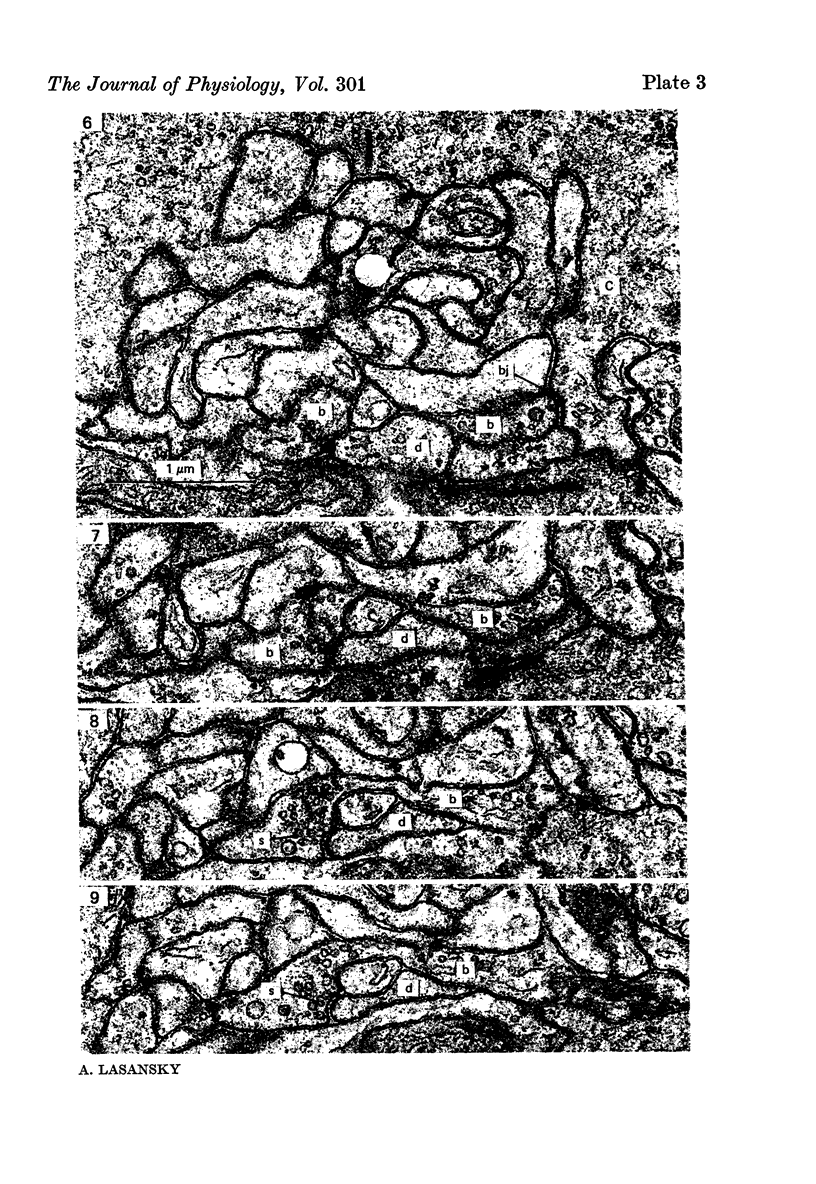
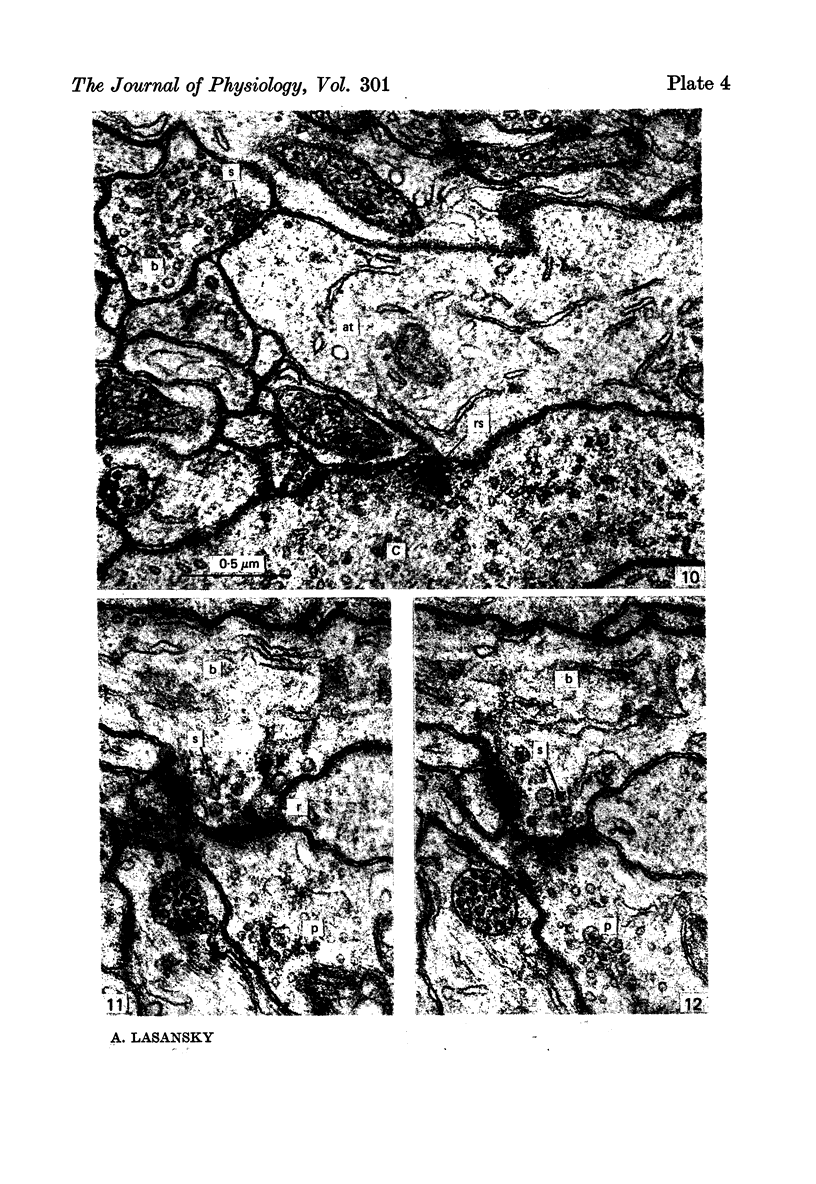
2. Horizontal cell dendrites make close membrane appositions (gap junctions) with one another and are post-synaptic to bipolar cell dendrites at presumed chemical synapses. On the other hand, there is no clear evidence that horizontal cell dendrites are presynaptic to any other neuronal processes at the outer plexiform layer, so that the output connexions of horizontal cell bodies remain a matter of speculation.
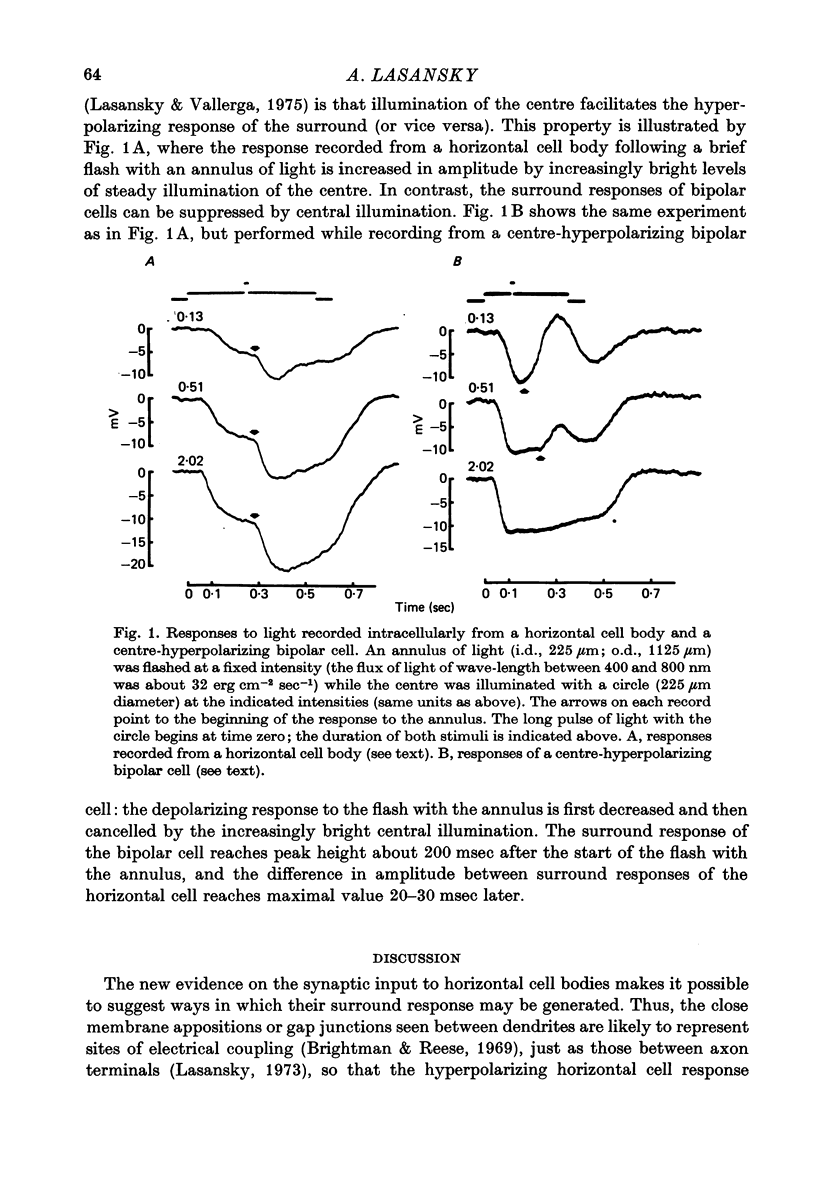
3. It is suggested that the bipolar cell input and the gap junctions between dendrites contribute, respectively, depolarizing and hyperpolarizing components to the responses of horizontal cell bodies to surround illumination. In addition, the facilitatory effect of central illumination on the surround response of horizontal cell bodies may result, although perhaps only partly, from observed properties of the surround response of bipolar cells.
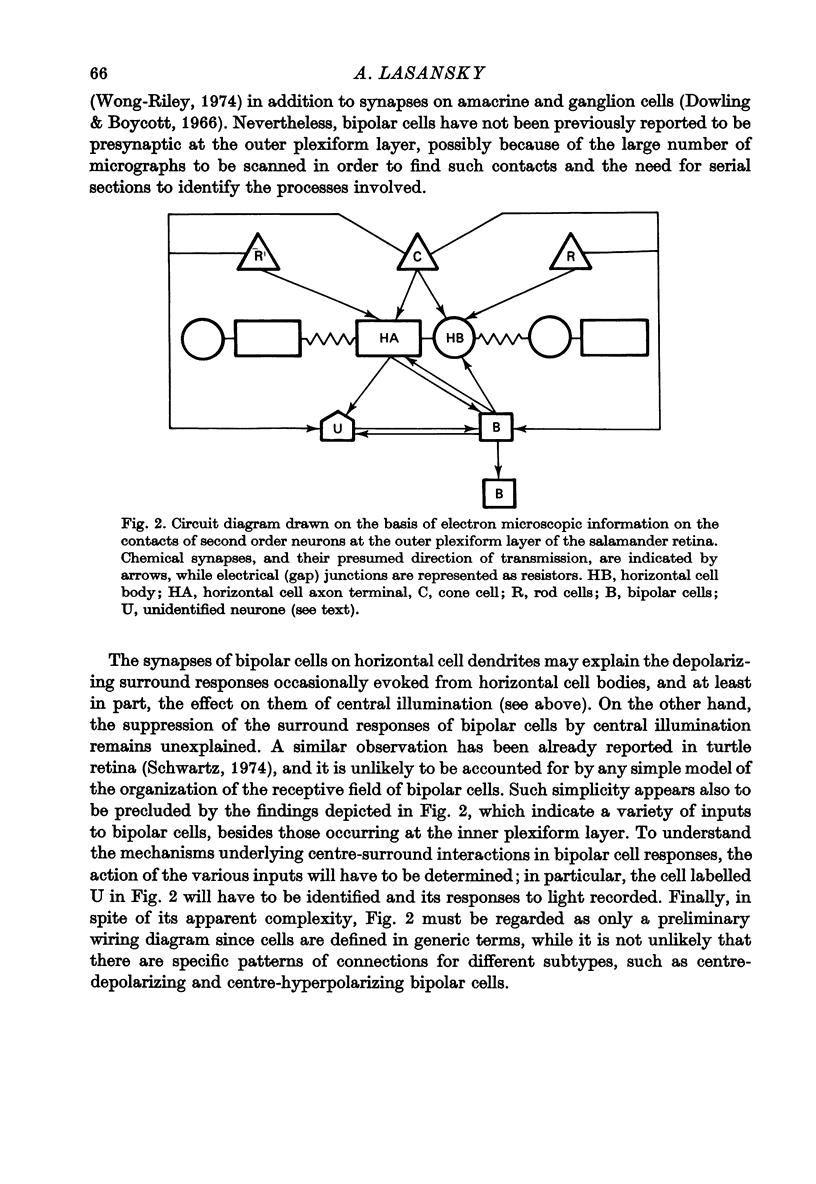
4. In the course of these observations, bipolar cells were found to be presynaptic at the outer plexiform layer not only to horizontal cell dendrites, but also to other bipolar cells, horizontal cell axon terminals and certain processes belonging to an as yet unidentified neurone.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Gray E. G., Guillery R. W. Synaptic morphology in the normal and degenerating nervous system. Int Rev Cytol. 1966;19:111–182. doi: 10.1016/s0074-7696(08)60566-5. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol. 1978 Dec;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasansky A., Vallerga S. Horizontal cell responses in the retina of the larval tiger salamander. J Physiol. 1975 Sep;251(1):145–165. doi: 10.1113/jphysiol.1975.sp011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACHEY L. D. Thin sections. I. A study of section thickness and physical distortion produced during microtomy. J Biophys Biochem Cytol. 1958 May 25;4(3):233–242. doi: 10.1083/jcb.4.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]