Abstract
1. Pyriformis slow (and sartorius twitch) fibres from Rana temporaria were studied with a three-micro-electrode voltage-clamp technique to obtain an approximate measurement of membrane current density at a fibre end. In most experiments, a modified Ringer solution containing 2H20 and 230 mM-sucrose was used to reduce movement. 2. Linear membrane properties of slow fibres obtained with this method are consistent with results from previous studies. Measured Cm (microF/cm2) increases with fibre diameter in a manner consistent with a tubular location of part of the fibre capacitance. 3. Voltage steps to -50mV and more positive potentials result in outward membrane currents in both slow and twitch fibres. These currents develop along similar sigmoid time courses and are blocked by tetraethylammonium (TEA+) ions. The reversal potential for delayed current channels in slow fibres varies with external K+ concentration, suggesting that the delayed current in slow fibres, as in twitch, is carried by K+ ions. 4. Maximum GK,GK, in slow fibres is an order of magnitude smaller than twitch fibres. The steady-state GK-V curve of slow fibres is very broad (e-fold for approximately 15 mV), saturating at very positive voltages, whereas the GK of twitch fibres varies more steeply with voltage. 5. No evidence of inward currents was seen in slow fibres during pulses of duration up to 96 msec. 6. Slow outward currents, which do not inactivate appreciably, are seen in slow fibres during long (10 sec) pulses. Tail currents following such long pulses are very slow. The reversal potential shifts to more positive values with increasing pulse duration.
Full text
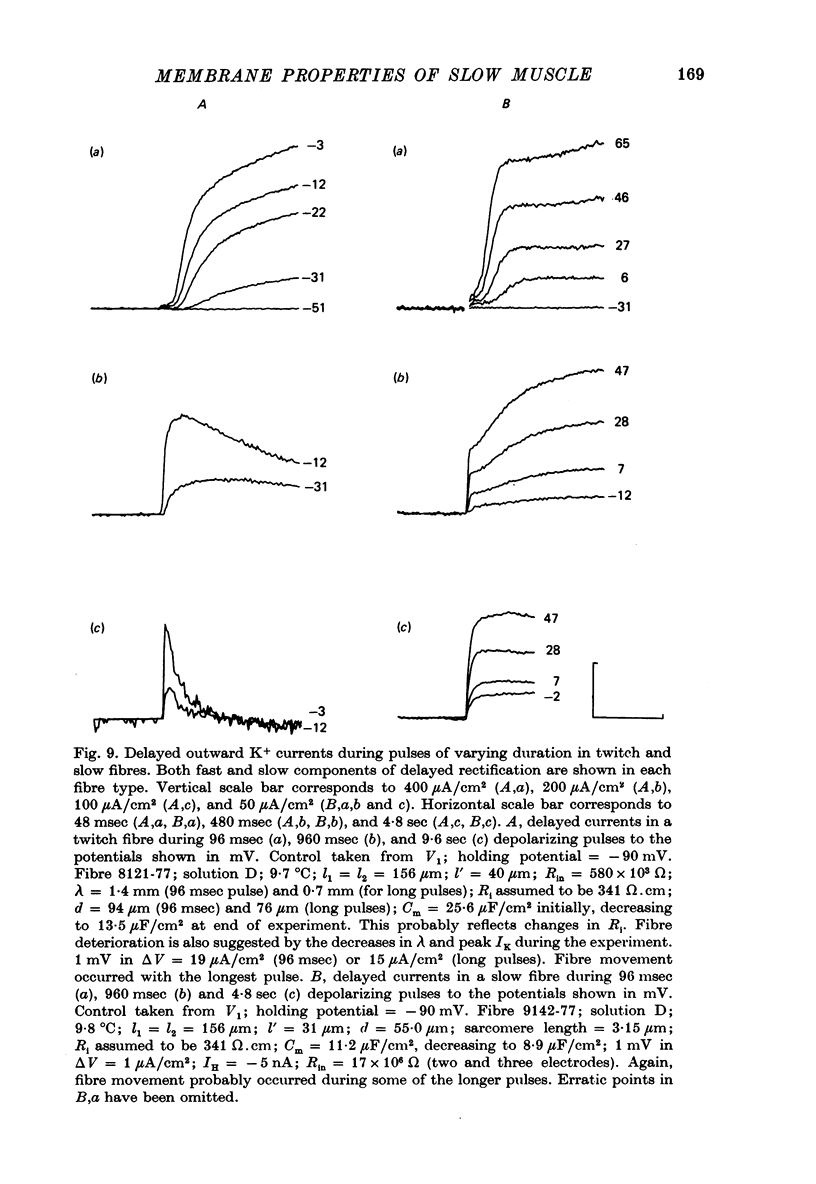
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
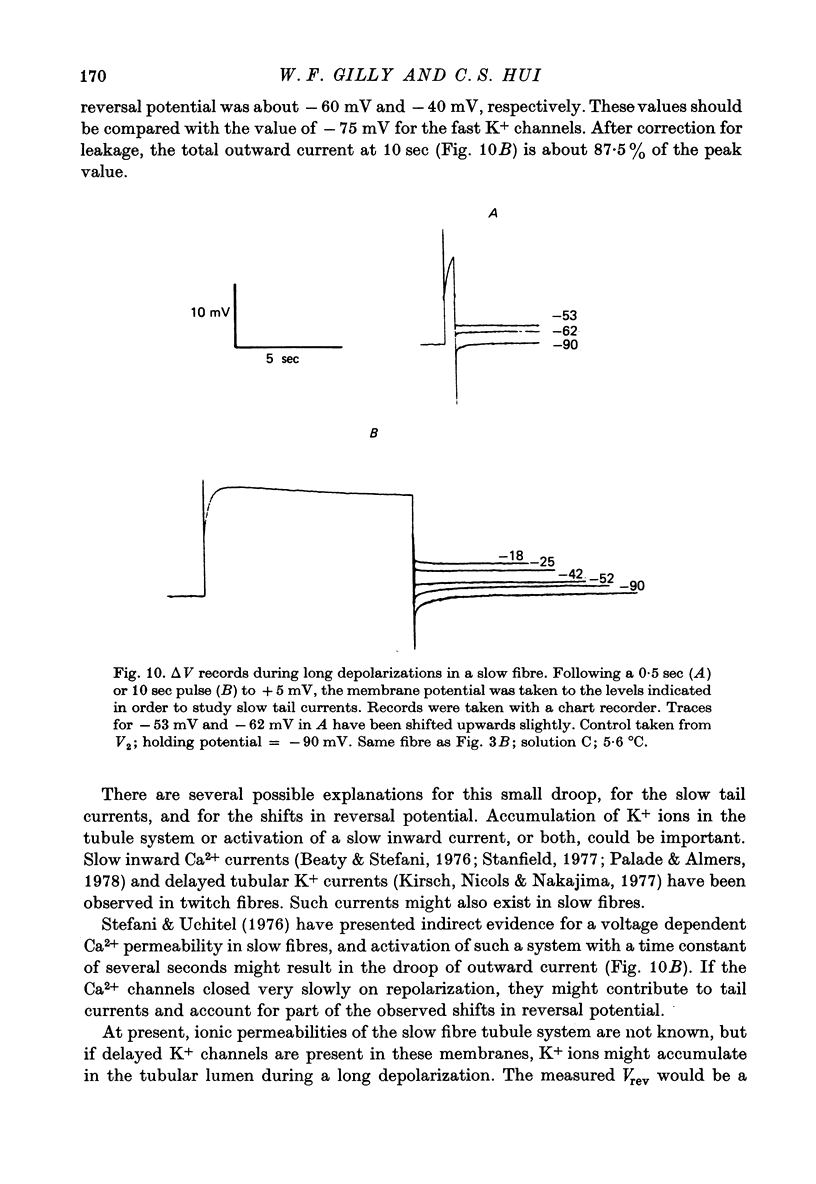
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. The membrane capacity of frog twitch and slow muscle fibres. J Physiol. 1965 Nov;181(2):324–336. doi: 10.1113/jphysiol.1965.sp007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Differential effects of tetracaine on delayed potassium channels and displacement currents in frog skeletal muscle. J Physiol. 1976 Nov;262(3):613–637. doi: 10.1113/jphysiol.1976.sp011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Palmieri G. Nerve fiber behaviour in heavy water under voltage-clamp. Biophysik. 1968 Aug 12;5(1):71–77. doi: 10.1007/BF01388134. [DOI] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Franzini-Armstrong C. The passive electrical properties of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1977 Apr;266(3):687–711. doi: 10.1113/jphysiol.1977.sp011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Franzini-Armstrong C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1975 Sep;250(3):513–539. doi: 10.1113/jphysiol.1975.sp011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARBY L., NORDQVIST P. The effect of deuterium oxide (heavy water) on conduction velocity in isolated frog nerve. Acta Physiol Scand. 1955 Oct 27;34(2-3):162–168. doi: 10.1111/j.1748-1716.1955.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Hui C. S. Mechanical activation in slow and twitch skeletal muscle fibres of the frog. J Physiol. 1980 Apr;301:137–156. doi: 10.1113/jphysiol.1980.sp013195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Hui C. S. Voltage-dependent charge movement in frog slow muscle fibres. J Physiol. 1980 Apr;301:175–190. doi: 10.1113/jphysiol.1980.sp013197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Nichols R. A., Nakajima S. Delayed rectification in the transverse tubules: origin of the late after-potential in frog skeletal muscle. J Gen Physiol. 1977 Jul;70(1):1–21. doi: 10.1085/jgp.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kuroda T. Effects of deuterium oxide on mechano-sensory receptor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4703–4705. doi: 10.1073/pnas.73.12.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasledov G. A., Zachar J., Zacharová D. The ionic requirements for the development of contracture in isolated slow muscle fibres of the frog. Physiol Bohemoslov. 1966;15(4):293–306. [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- SVENSMARK O. The effect of deuterium oxide on the mechanical properties of muscle. Acta Physiol Scand. 1961 Sep;53:75–84. doi: 10.1111/j.1748-1716.1961.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Uchitel O. D. Potassium and calcium conductance in slow muscle fibres of the toad. J Physiol. 1976 Feb;255(2):435–448. doi: 10.1113/jphysiol.1976.sp011288. [DOI] [PMC free article] [PubMed] [Google Scholar]


