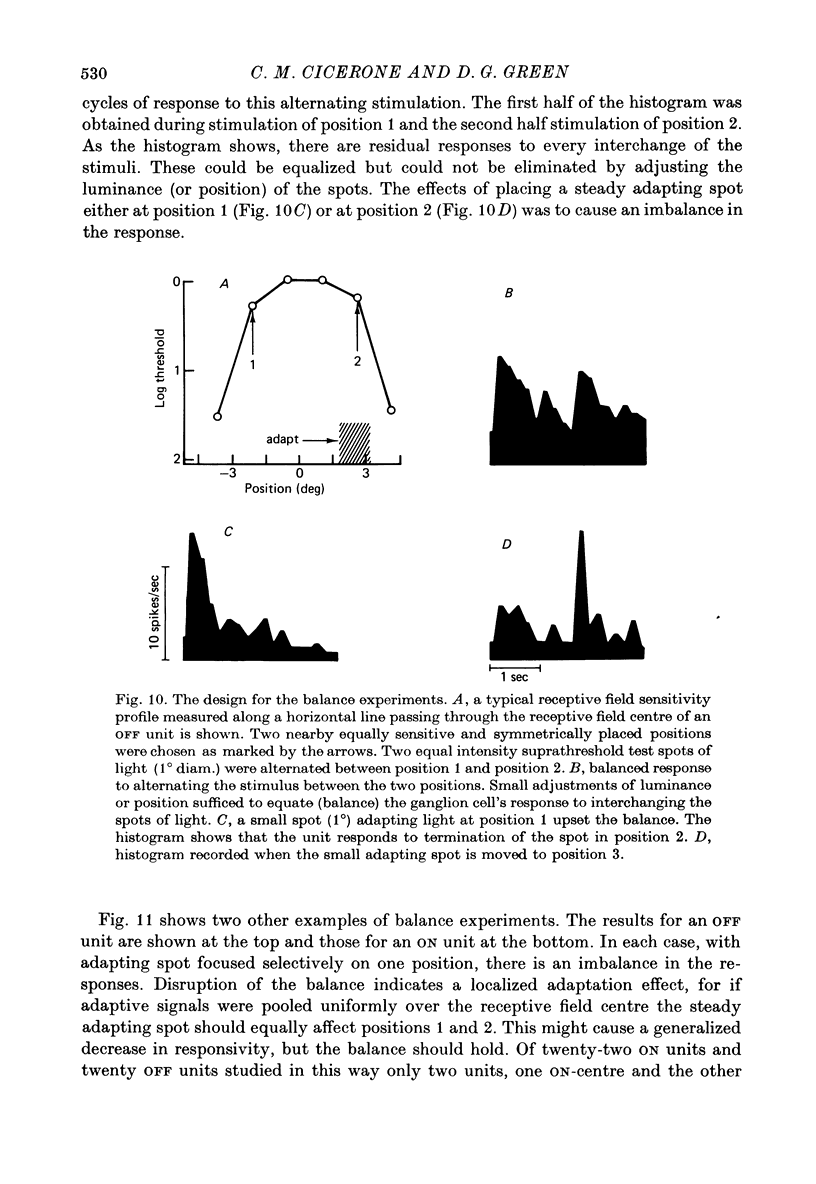
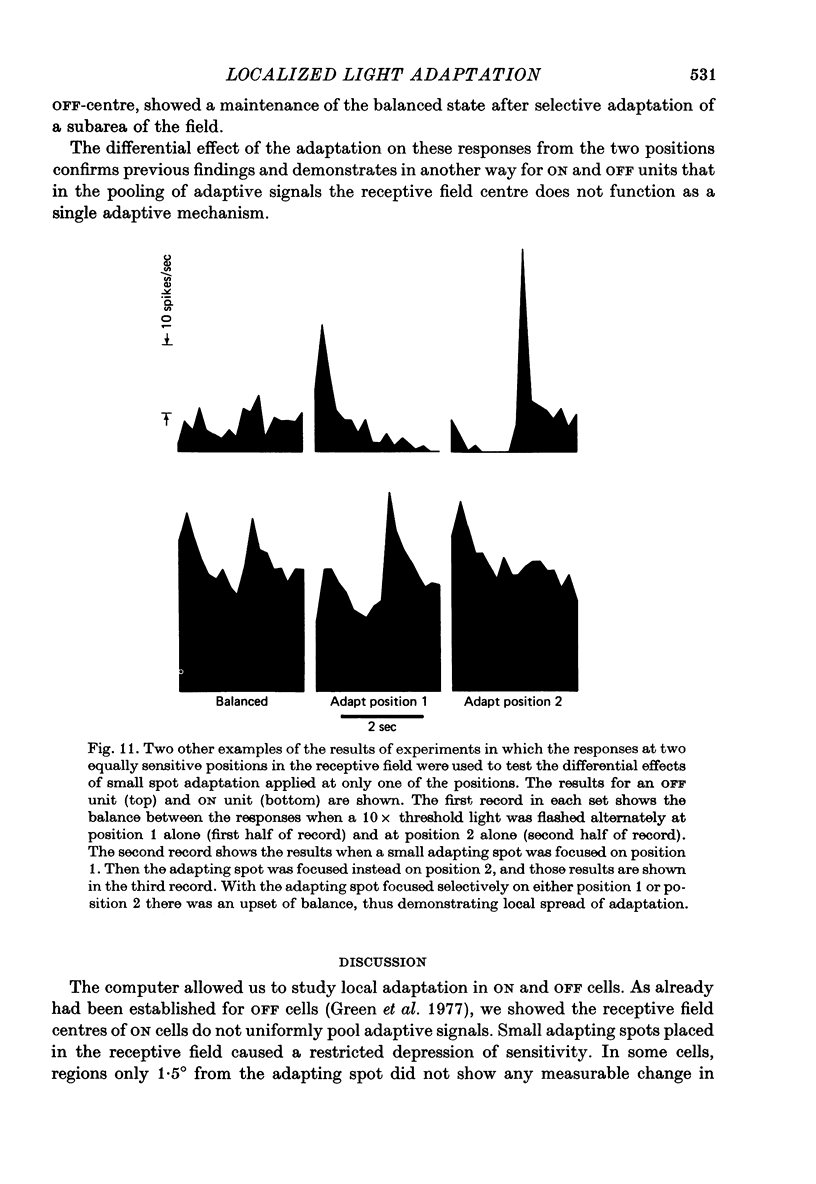
Abstract
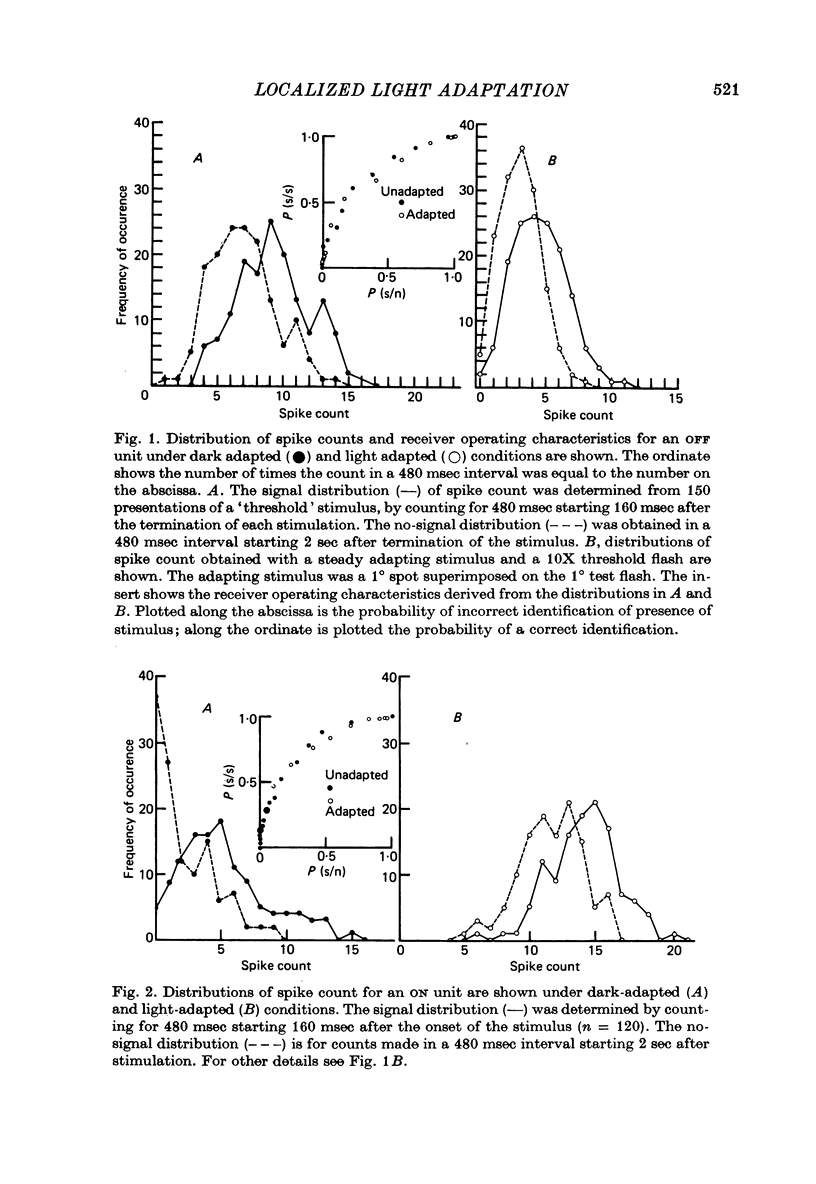
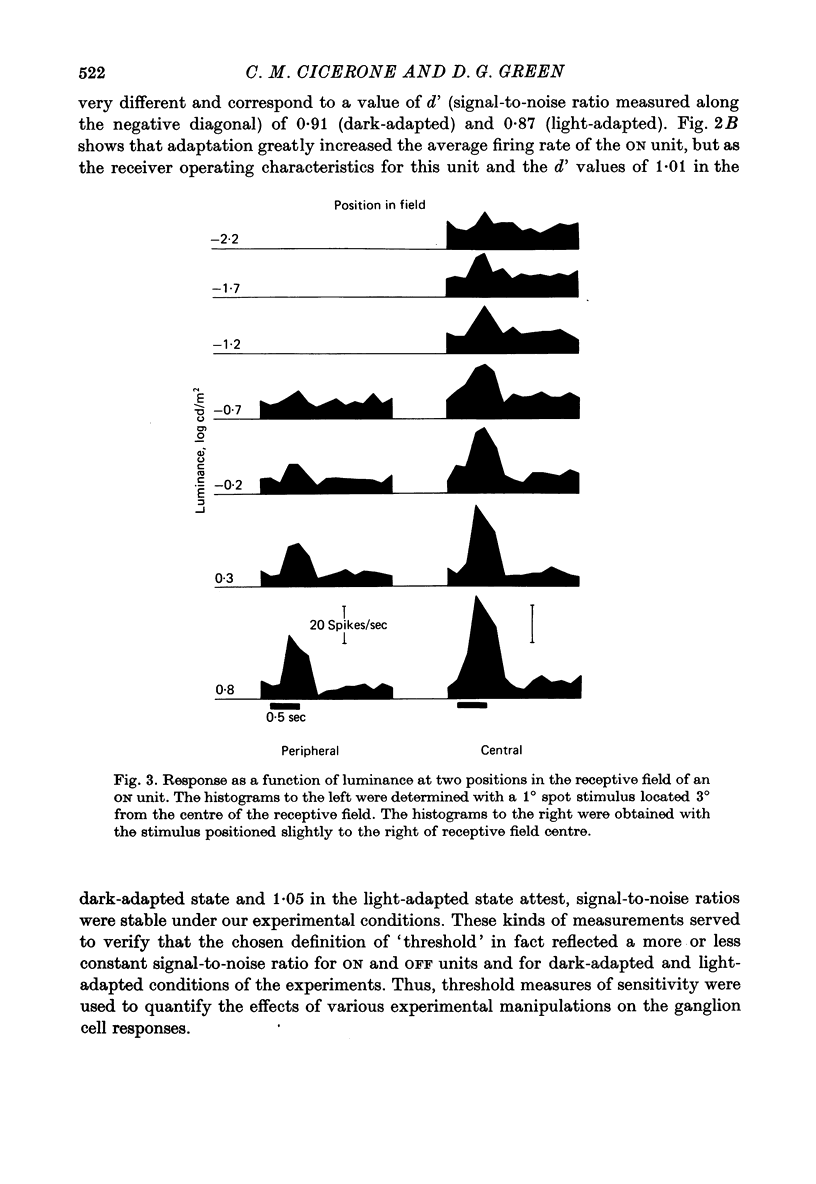
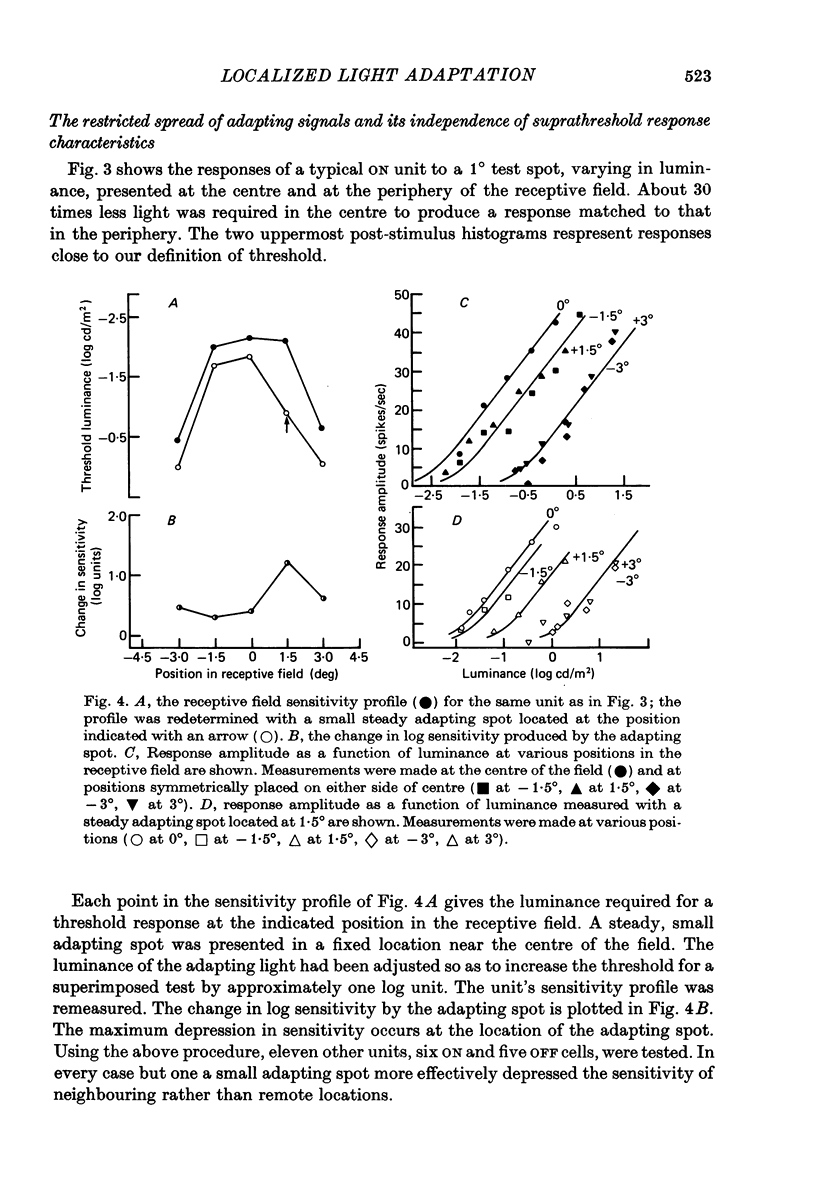
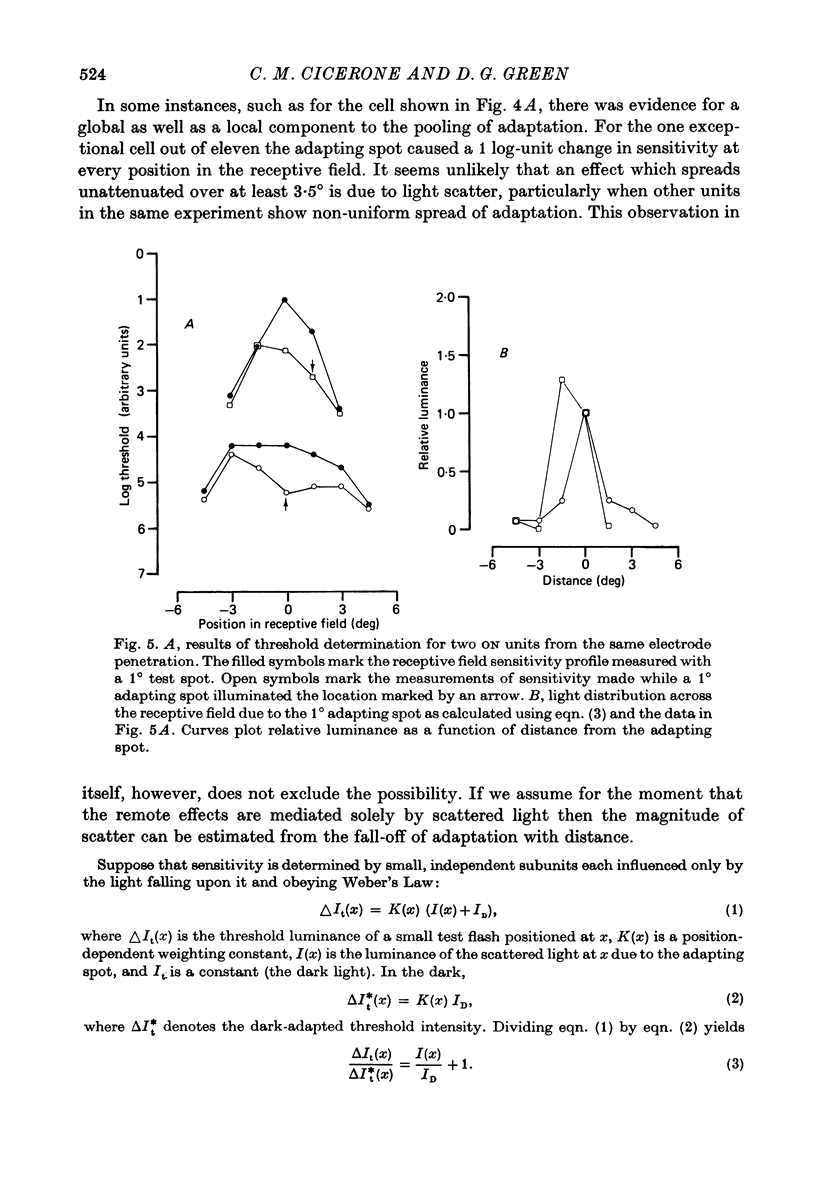
1. Responses from axons of single retinal ganglion cells in the rat's optic tract were used to measure the pooling of adaptive signals within the cells' receptive field. Computer-aided analyses of response measurements were used to evaluate sensitivity at a number of field locations. 2. A small adapting spot caused a localized decrease in sensitivity within the receptive field centre of ON- and OFF-centre ganglion cells. 2. The functions describing response versus test luminance were similar in shape for all test and adaptation configurations. This assured that, using a fixed criterion response, sensitivity determinations could be made just as well in any receptive field location and under any of the experimental conditions. 4. A concentric surround, antagonistic to the receptive field centre, was readily apparent only under conditions of light adaptation. Experiments on the local effects of small adapting spots, conducted with selective surround adaptation, showed that the non-uniform spread of adaptation within the receptive field centre was not linked to surround intrusion. 5. The possibility that the photopic mechanism intruded to contaminate these results was considered and rejected. 6. When a suprathreshold spot was alternated between two equally sensitive positions, the ganglion cell gave an approximately balanced response. An upset of this balance was produced by placing a small adapting spot at either position, thus demonstrating, in another way, the non-uniform spread of adaptation within the receptive field centre. 7. It is concluded that significant pooling of adaptation effects occurs prior to the combination of influences which contribute to the centre response of a ganglion cell.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Andrews D. P. Sensitivity of receptors and receptor "pools". J Opt Soc Am. 1967 Jun;57(6):837–838. doi: 10.1364/josa.57.000837. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone C. M. Cones survive rods in the light-damaged eye of the albino rat. Science. 1976 Dec 10;194(4270):1183–1185. doi: 10.1126/science.996550. [DOI] [PubMed] [Google Scholar]
- Cicerone C. M., Green D. G. Control of eye movements while recording from single units in the pigmented rat. Vision Res. 1977;17(8):985–987. doi: 10.1016/0042-6989(77)90076-1. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn T. E., Green D. G., Tanner W. P., Jr Receiver operating characteristic analysis. Application to the study of quantum fluctuation effects in optic nerve of Rana pipiens. J Gen Physiol. 1975 Nov;66(5):583–616. doi: 10.1085/jgp.66.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G. Light adaptation in the rat retina: evidence for two receptor mechanisms. Science. 1971 Nov 5;174(4009):598–600. doi: 10.1126/science.174.4009.598. [DOI] [PubMed] [Google Scholar]
- Green D. G., Tong L., Cicerone C. M. Lateral spread of light adaptation in the rat retina. Vision Res. 1977;17(3):479–486. doi: 10.1016/0042-6989(77)90042-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Noell W. K., Walker V. S., Kang B. S., Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966 Oct;5(5):450–473. [PubMed] [Google Scholar]
- Partridge L. D., Brown J. E. Receptive fields of rat retinal ganglion cells. Vision Res. 1970 Jun;10(6):455–460. doi: 10.1016/0042-6989(70)90002-7. [DOI] [PubMed] [Google Scholar]
- Powers M. K., Green D. G. Single retinal ganglion cell responses in the dark-reared rat: grating acuity, contrast sensitivity, and defocusing. Vision Res. 1978;18(11):1533–1539. doi: 10.1016/0042-6989(78)90008-1. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. THE SENSITIVITY OF RODS UNDER ILLUMINATION. J Physiol. 1965 May;178:141–160. doi: 10.1113/jphysiol.1965.sp007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson J. G., Enroth-Cugell C. Light distribution in the cat's retinal image. Vision Res. 1978;18(2):159–173. doi: 10.1016/0042-6989(78)90181-5. [DOI] [PubMed] [Google Scholar]
- Tong L., Green D. G. Adaptation pools and excitation receptive fields of rat retinal ganglion cells. Vision Res. 1977;17(10):1233–1236. doi: 10.1016/0042-6989(77)90160-2. [DOI] [PubMed] [Google Scholar]


