Abstract
The cecropin-like bactericidal peptide Hp(2-20) from Helicobacter pylori induces activation of the NADPH oxidase in human neutrophils via formyl peptide receptor-like 1 (FPRL1) (J. Bylund, T. Christophe, F. Boulay, T. Nyström, A. Karlsson, and C. Dahlgren, Antimicrob. Agents Chemother. 45:1700-1704, 2001). Here we investigated the ability of bacterial lipopolysaccharide (LPS) to prime this response. Neutrophils treated with LPS for 30 min at 37°C produced substantially more superoxide anion than control cells upon stimulation with Hp(2-20). Hence, LPS primed the cells for subsequent stimulation through FPRL1. To study the molecular background of this priming phenomenon, we measured the degrees of granule mobilization and concomitant receptor upregulation to the cell surface in LPS-treated cells. Exposure of complement receptors 1 and 3 as well as the formyl peptide receptor (FPR) was markedly increased after LPS treatment. Since approximately 60% of the gelatinase granules were mobilized while the specific granules were retained, we hypothesized that the gelatinase granules were potential stores of FPRL1. The presence of FPRL1 mainly in the gelatinase granules was confirmed by Western blotting of subcellular fractions of resting neutrophils. These results suggest that the mechanism behind the LPS-induced priming of FPRL1-mediated responses lies at the level of granule (receptor) mobilization.
The innate immune defense toward microorganisms is largely dependent on neutrophil granulocytes. Neutrophil effector functions include the production of oxygen radicals that have bactericidal functions and that are potentially tissue destructive (4). Hence, tight regulation of the radical-producing enzyme system, the NADPH oxidase, is of major importance in producing an effective defense toward infection without causing pathological destruction of surrounding tissues. Helicobacter pylori, the bacterial pathogen associated with gastritis and peptic ulcers, is highly successful in establishing infection in the human gastric mucosa, a process typically associated with massive infiltration of inflammatory cells and tissue destruction (1). Colonization of the mucosa is suggested to be facilitated by H. pylori-produced cecropin-like peptides with antibacterial properties, giving the microbe a competitive advantage over other bacteria (39). It was shown earlier that such a peptide, Hp(2-20), in addition to being bactericidal, is a neutrophil chemoattractant that activates these cells to release reactive oxygen species generated by the NADPH oxidase (11). These effects are mediated through the binding of Hp(2-20) to the promiscuous, G-protein-linked formyl peptide receptor-like 1 (FPRL1) (11). This receptor was originally cloned from human phagocytes by low-stringency hybridization of a cDNA library with the formyl peptide receptor (FPR) sequence and was initially defined as an orphan receptor (37, 49). In addition to Hp(2-20), several other ligands for FPRL1 have been identified in the past few years (12, 16, 20, 22, 34, 42, 43). These ligands do not contain any sequence homologies but have in common the fact that binding to FPRL1 results in a G-protein-mediated signaling cascade leading to cell activation. Thus, FPRL1 signaling is very similar to that of the FPR, consistent with the fact that the receptors share 69% sequence identity.
Most studies of neutrophil activation have been conducted on cells isolated from peripheral blood. However, neutrophils exert their function in vivo mainly after entry into inflammatory sites. Concomitant with exudation from the bloodstream, the cells become primed (i.e., hyperresponsive) with respect to the NADPH oxidase activity induced by inflammatory mediators, e.g., the chemoattractant formylmethionyl-leucyl-phenylalanine (fMLF) (25) and galectin-3 (29). The priming phenomenon has been described for many settings in neutrophil activation processes, prominent examples being the effect of bacterial lipopolysaccharide (LPS) (27) and the adhesion-related priming of tumor necrosis factor-induced responses (38). Many priming mechanisms have been suggested, and it is reasonable to believe that different mechanisms, alone or in concert, may be the causes of different priming events (14, 23, 28). Neutrophils that have encountered bacterial LPS are primed with respect to the oxidative response; i.e., LPS per se does not activate the NADPH oxidase but induces hyperresponsiveness to other stimuli (2, 21, 27, 44, 46). The prevailing view proposes that the mechanism for this LPS-induced priming involves alterations in intracellular signaling (e.g., changes in the levels of various secondary messengers) and/or direct effects on the NADPH oxidase (27). We as well as others have proposed that one important mechanism behind the primed state induced during neutrophil extravasation is the mobilization of intracellular granules, endowing the plasma membrane with new receptors (29). It was recently shown that such mobilization of specific receptors can explain priming vis-à-vis fMLF as well as galectin-3 (2).
Here we investigate the effect of LPS pretreatment on the neutrophil response to Hp(2-20) and the concomitant mobilization of granules. The results show that LPS primes the neutrophil response to Hp(2-20) and that mobilization of gelatinase granules, storing a considerable pool of the neutrophil receptor for Hp(2-20) (FPRL1), is a likely cause for the primed state.
MATERIALS AND METHODS
Isolation of human neutrophils.
Neutrophils were isolated from buffy coats from healthy blood donors by using dextran sedimentation and Ficoll-Paque gradient centrifugation (9). The cells were washed and resuspended (107/ml) in Krebs-Ringer phosphate buffer containing glucose (10 mM), Ca2+ (1 mM), and Mg2+ (1.5 mM) (KRG; pH 7.3). This isolation procedure allows for cells to be isolated with minimal mobilization effects (3).
Neutrophil cytoplasts were prepared by the method of Roos et al. (40) as described earlier (17).
Priming with LPS.
LPS from Escherichia coli serotype O111:B4 was dissolved in KRG to 1 mg/ml and sonicated to prepare a homogeneous solution. Cells (107/ml) were incubated in the presence or absence of LPS (10 μg/ml) (this relatively high concentration is required to induce priming in the absence of serum) at 4 or 37°C for 30 min and were directly used for NADPH oxidase activation studies or marker analysis.
NADPH oxidase activity.
The superoxide anion produced by NADPH oxidase was determined by using an isoluminol-enhanced chemiluminescence (CL) system (19). The CL activity was measured by using a six-channel Biolumat LB 9505 apparatus (Berthold Co., Wildbad, Germany) with disposable 4-ml polypropylene tubes and a 0.36-ml reaction mixture containing 2 × 105 neutrophils or cytoplasts. The tubes were equilibrated in the Biolumat apparatus for 5 min at 37°C, after which the stimulus (0.04 ml) was added. The emitted light was measured continuously. By a direct comparison of the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c and SOD-inhibitable CL, 7.2 × 107 cpm was found to correspond to the production of 1 nmol of superoxide (a millimolar extinction coefficient for cytochrome c of 21.1 was used).
Preparation of a polyclonal anti-FPRL1 antibody.
A polyclonal antibody against the C terminus (the terminal 10 amino acid residues; PPAETELQAM) of FPRL1 was raised in rabbits. The antibody was affinity purified and used in Western blots to identify FPRL1. The specificity of the antibody was determined by its ability to stain permeabilized cells stably expressing either of the members of the FPR family (13, 16). The antibody was found to stain cells expressing FPRL1 or FPRL2 (expressed only in monocytes and with the C-terminal sequence PPEETELQAM) but not cells expressing FPR (with the C-terminal sequence LPSAEVELQAK).
Subcellular fractionation.
Subcellular fractionation was performed in principle as described by Borregaard et al. (8). In brief, neutrophils isolated from buffy coats were treated with the serine protease inhibitor diisopropyl fluorophosphate (8 μM) and disintegrated by nitrogen cavitation (Parr Instruments Co., Moline, Ill.), and the postnuclear supernatant was centrifuged on Percoll gradients. To determine whether FPRL1 is localized in the light membranes, i.e., the plasma membranes and secretory vesicles, a Percoll gradient was designed to separate these organelles from the specific granules and azurophil granules. To separate the plasma membranes from the secretory vesicles, a flotation gradient was used as previously described (15). The gelatinase granules were separated from the classical specific granules as described by Kjeldsen et al. (31). The gradients were collected in 1.5-ml fractions by aspiration from the bottom of the centrifuge tube, and the localization of subcellular organelles in the gradients was determined by marker analysis of the fractions (see below). The fractions containing isolated secretory vesicles, plasma membranes, gelatinase granules, and specific granules were pooled and centrifuged (100,000 × g, 90 min, 4°C), and the pellets were resuspended.
SDS-PAGE and Western blotting.
Percoll gradient fractions or isolated granules and membranes were diluted in nonreducing sample buffer, boiled for 5 min, and applied to sodium dodecyl sulfate (SDS)-10% polyacrylamide gels (32) in volumes corresponding to 5 × 106 cells. After polyacrylamide gel electrophoresis (PAGE), the proteins were transferred to polyvinylidene difluoride membranes by using a Tris-glycine buffer system (10). The polyvinylidene difluoride membranes were blocked overnight at 4°C in phosphate-buffered saline-Tween containing 1% bovine serum albumin and 1% milk (wt/vol) (blocking solution). After two washes for 5 min each time in phosphate-buffered saline-Tween, the blots were incubated with anti-FPRL1 antibody (diluted 1/200 in blocking solution) for 1 h at room temperature. Nonbound antibody was removed by washing (two washes for 5 min each time), and the blots were incubated with horseradish peroxidase-labeled anti-rabbit immunoglobulin G (diluted 1/1,000 in blocking solution) for 1 h at room temperature. Bound antibody was detected by adding a peroxidase substrate (VIP-kit; Vector Laboratories, Burlingame, Calif.).
Marker analysis.
To localize the organelles in the Percoll gradients, each fraction was assessed for contents of myeloperoxidase (marker for azurophil granules; measured by hydrolysis of o-phenylenediamine), vitamin B12 binding protein (marker for specific granules) (26), gelatinase (marker for gelatinase and specific granules; measured by using an enzyme-linked immunosorbent assay) (30), latent alkaline phosphatase (marker for secretory vesicles; measured by hydrolysis of p-nitrophenyl phosphate in the presence and absence of Triton X-100), and HLA class I (marker for the plasma membrane) (5).
The mobilization of subcellular organelles after treatment of the cells with LPS was monitored by measuring the exposure of complement receptor 1 (CR1; marker for secretory vesicles), CR3 (marker for secretory vesicles and for gelatinase and specific granules), and FPR on the neutrophil surface. Exposure of CR1 was measured by labeling the cells with mouse anti-human CD35 (Dakopatts M0710; 10 μl for a cell pellet of 106 cells) and subsequent binding of fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (Dako F0479; 1/2,000). To measure CR3 exposure, the cells were labeled with a phycoerythrin-conjugated monoclonal antibody specific for CD11b (Dako M741; 10 μl for a cell pellet of 106 cells) (35). The cells were examined with a FACScan apparatus (Becton Dickinson, Mountain View, Calif.). The amounts of fMLF receptors on the cell surface were determined by incubating neutrophils with radiolabeled fMLF in the presence or absence of excess unlabeled fMLF as described previously (3). The release of gelatinase and vitamin B12 binding protein into the supernatant was assessed as described above.
Peptides, reagents, and antibodies.
The peptide Hp(2-20), AKKVFKRLEKLFSKIQNDK, was synthesized and purified (>95%) at Innovagen (Lund, Sweden), dissolved in water, and stored at −70°C until use. The hexapeptide Trp-Lys-Tyr-Met-Val-d-Met-NH2 (WKYMVm) was synthesized and purified by high-pressure liquid chromatography at Alta Bioscience (University of Birmingham, Birmingham, United Kingdom). LPS (E. coli serotype O111:B4; L-2630), fMLF, and isoluminol were obtained from Sigma Chemical Co. (St. Louis, Mo.). [3H]fMLF was supplied by Du Pont NEN (Boston, Mass.). Catalase, SOD, and horseradish peroxidase were purchased from Boehringer Mannheim (Mannheim, Germany). o-Phenylenediamine was obtained from Dako (Glostrup, Denmark). Dextran and Ficoll-Paque were obtained from Pharmacia (Uppsala, Sweden). 57Co-labeled vitamin B12 was supplied by Amersham Laboratories (Amersham, Buckinghamshire, England). Antibodies for the gelatinase enzyme-linked immunosorbent assay were kind gifts from Lars Kjeldsen and Niels Borregaard, Copenhagen, Denmark. All other antibodies were obtained from Dako.
RESULTS
LPS-induced priming of the neutrophil oxidative response to Hp(2-20).
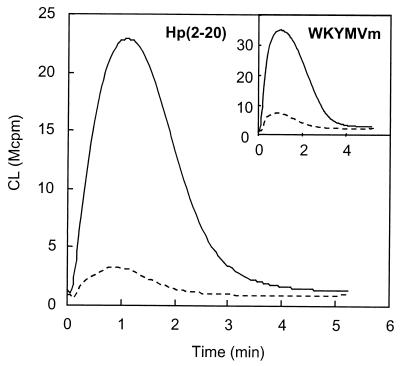
We first measured the extracellular release of oxygen radicals from LPS-primed and nonprimed neutrophils in response to Hp(2-20). Neutrophils pretreated with LPS for 30 min at 37°C exhibited increased extracellular release of oxygen radicals in response to the peptide compared to the response seen for nonprimed cells (Fig. 1). The response to the chemotactic peptide WKYMVm, which, by analogy to Hp(2-20), acts through FPRL1 (16), was also primed by LPS (Fig. 1, inset).
FIG. 1.
Hp(2-20)-induced release of oxygen radicals in LPS-primed and nonprimed neutrophils. Cells were preincubated for 30 min at 37°C in the presence (solid line) or absence (broken line) of LPS (10 μg/ml) and then stimulated with Hp(2-20) (50 μM) or WKYMVm (10 nM; inset). The extracellular release of superoxide anion was measured by isoluminol-amplified CL, and the responses are given as 106 counts per minute (Mcpm). Representative kinetics of four or five independent experiments are shown.
Neutrophil priming induced by bacterial LPS is a time- and temperature-dependent phenomenon. To establish a reference for comparison with the literature (2, 21, 27, 44, 46), we also measured fMLF-induced activation in LPS-primed and nonprimed neutrophils. In agreement with previous studies, pretreatment of the cells with LPS for 30 min at 37°C resulted in a markedly enhanced response to fMLF (data not shown), while there was no difference in superoxide production between LPS-primed and nonprimed neutrophils when stimulated with the protein kinase C activator phorbol myristate acetate (data not shown).
Effect of LPS-induced priming on neutrophil cytoplasts.
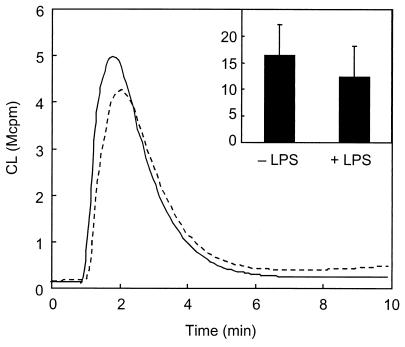
It was recently shown that priming of the fMLF response by LPS involves degranulation and receptor upregulation to the cell surface (2). In order to investigate the influence of granule-localized FPRL1 on LPS-induced priming, we used neutrophil cytoplasts. Cytoplasts consist of organelle-free cytoplasm surrounded by plasma membrane and have successfully been used to assess the role of neutrophil granules in specific cellular responses (18, 24). Hp(2-20) was a potent activator of NADPH oxidase in neutrophil cytoplasts, but the oxidase activity was not increased by LPS treatment (Fig. 2). Likewise, LPS failed to prime the cytoplasts when stimulated with fMLF and WKYMVm (data not shown). These results suggest that the granules are of importance for the priming phenomenon.
FIG. 2.
Hp2-20-induced release of oxygen radicals in LPS-primed and nonprimed neutrophil cytoplasts. Cytoplasts were preincubated for 30 min at 37°C in the presence (solid line) or absence (broken line) of LPS (10 μg/ml) and then stimulated with Hp(2-20) (50 μM). The extracellular release of superoxide anion was measured by isoluminol-amplified CL, and the responses are given as 106 counts per minute (Mcpm). The kinetics of a representative experiment and the mean peak value (and standard deviation) of five experiments (inset) are shown.
LPS-induced mobilization of subcellular organelles.
Receptors for many neutrophil activators are stored in mobilizable granules in peripheral blood neutrophils (7). The results obtained with cytoplasts indicate that FPRL1 can be mobilized from intracellular granules as well. To investigate this possibility in more detail, we measured the cell surface exposure of known membrane components (CR1, CR3, and FPR) and the extracellular release of granule contents (gelatinase and vitamin B12 binding protein) with and without LPS treatment.
Neutrophils incubated at 37°C with LPS showed a moderate increase in the surface expression of CR1 and CR3 compared to cells incubated at 37°C without LPS (Table 1). A more dramatic increase was seen in the release of gelatinase (from 13 to 34%) as well as in the exposure of FPR (from 128 to 278%). The release of vitamin B12 binding protein from specific granules increased only from 3 to 5%. Hence, the major effect of LPS with regard to degranulation was that the gelatinase granules were mobilized while the specific granules were retained (Table 1). Since gelatinase is approximately equally distributed between the gelatinase granules and the specific granules, the percent mobilization of gelatinase granules can be estimated as follows: 2 × (percent release of gelatinase − percent release of vitamin B12 binding protein). Thus, about 60% of the gelatinase granules were mobilized in the presence of LPS.
TABLE 1.
Mobilization of granule markers upon treatment with LPS
| Condition(s) | Mean ± SD % expression of:
|
||||
|---|---|---|---|---|---|
| CR1a | CR3a | FPRa | Gelatinaseb | Vitamin B12 binding proteinb | |
| 4°C | 100 | 100 | 100 | 1 ± 2 | 0 |
| 37°C | 265 ± 75 | 223 ± 44 | 128 ± 49 | 13 ± 8 | 3 ± 2 |
| 37°C + LPS | 350 ± 137 | 288 ± 66 | 278 ± 59 | 34 ± 11 | 5 ± 3 |
Relative to the control; n = 3 to 5.
Relative to total; n = 4 to 6.
LPS-induced priming of FPRL1-mediated responses is accompanied by mobilization of the gelatinase granules. Thus, a plausible explanation for the priming phenomenon is that FPRL1 is stored in these intracellular organelles and become upregulated to the cell surface upon LPS treatment. We therefore investigated the subcellular localization of FPRL1 in resting neutrophils.
Subcellular localization of FPRL1.
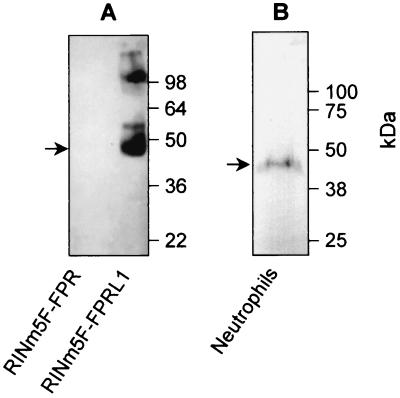
To study the presence of FPRL1 in subcellular compartments, a polyclonal anti-FPRL1 antibody was used. The antibody specificity was tested by Western blot analysis with lysates of RINm5F cells overexpressing either FPR or FPRL1 (13, 33). A major protein with a molecular mass of about 45 kDa was detected in the lysate of RINm5F cells expressing FPRL1 (Fig. 3A). A species with a higher mass (∼100 kDa), which may represent a dimeric form of FPRL1, was also observed. No band was detected in the lysate of RINm5F cells expressing FPR (Fig. 3A). In human peripheral blood neutrophils, the antibody raised against FPRL1 recognized a major 43- to 45-kDa protein (Fig. 3B).
FIG. 3.
Presence of FPRL1 in neutrophils. (A) The specificity of the anti-FPRL1 antibody was confirmed by Western blot analysis of lysates (106 cell equivalents) of RINm5F cells overexpressing either FPR or FPRL1. (B) A whole neutrophil lysate (5 × 107 cell equivalents) was analyzed by Western blotting for the presence of FPRL1 by using the anti-FPRL1 antibody. The presence of FPRL1 is indicated by arrows, and molecular sizes are given in kilodaltons. The predicted molecular mass of FPRL1 is 39 kDa, suggesting that the protein is glycosylated both in RINm5F cells and in neutrophils.
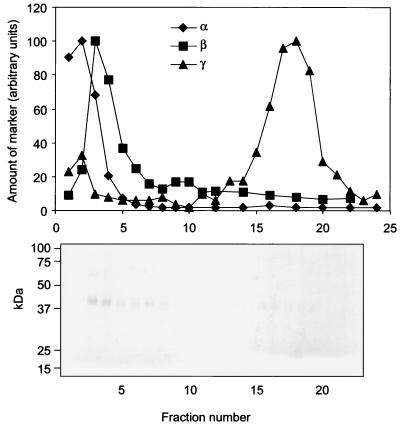
To determine the subcellular localization of FPRL1, resting neutrophils were disintegrated and fractionated on discontinuous Percoll gradients. In the Percoll gradient fractions, three subcellular entities were identified (Fig. 4): plasma membranes and secretory vesicles (the γ band), specific granules (the β band), and azurophil granules (the α band). Immunoblotting of the fractions with the anti-FPRL1 antibody revealed no FPRL1 in the upper part of the gradient, corresponding to the plasma membranes and secretory vesicles (Fig. 4). Instead, the major fraction of FPRL1 was found in the specific granules (the β band; fractions 3 and 4), whereas no FPRL1 was detected in the azurophil granules (the α band; fractions 1 and 2).
FIG. 4.
Subcellular localization of FPRL1. (Top) A postnuclear supernatant of disrupted neutrophils (109 cells) was fractionated on a discontinuous two-layer Percoll density gradient containing 3 ml of high-density Percoll (1.12 g/ml) and 26 ml of low-density Percoll (1.05 g/ml). Three bands were visible and were denoted α, β, and γ in order of decreasing density. Cytosolic proteins were present in fraction 22. Fractions (1.5 ml) were analyzed for myeloperoxidase (marker for azurophil granules; diamonds), vitamin B12 binding protein (marker for specific granules; squares), and alkaline phosphatase (marker for secretory vesicles and plasma membranes; triangles). (Bottom) From each fraction, 5 μl was also subjected to SDS-PAGE under nonreducing conditions with 10% (wt/vol) polyacrylamide gels and immunoblotting with anti-FPRL1 antibody.
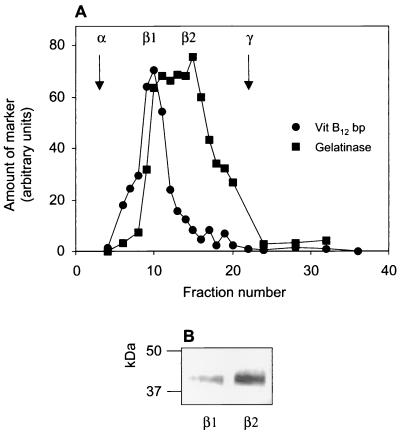
To investigate the subcellular localization of FPRL1 in more detail, the postnuclear material was fractionated on modified Percoll gradients. Since the major part of FPRL1 was localized in the β fraction, containing both the specific and the gelatinase granules, the postnuclear material was fractionated on a three-step Percoll gradient (31), which separates these two granule types. As determined from the marker distribution profiles, the specific granules were found in the denser band, β1, as indicated by the presence of both vitamin B12 binding protein and gelatinase (Fig. 5). The gelatinase granules were found in the lighter band, β2, as indicated by the presence of gelatinase but no vitamin B12 binding protein (31). The peak fractions containing the specific granules and the gelatinase granules were pooled separately. The granules were pelleted by centrifugation and analyzed by SDS-PAGE (1.5 × 107 cell equivalents) for detection of FRPL1 by immunoblotting. FPRL1 was found in both samples, but the amount was somewhat higher in the fraction enriched in gelatinase granules (Fig. 5).
FIG. 5.
Presence of FPRL1 in gelatinase granules and specific granules. A postnuclear supernatant of disrupted neutrophils was fractionated on a three-step Percoll density gradient. (A) Fractions were analyzed for vitamin B12 binding protein (Vit B12 bp) (specific granule marker) and gelatinase (marker for gelatinase granules and specific granules). The positions in the gradient of the azurophil granules (α) and the plasma membranes and secretory vesicles (γ) are indicated by arrows. (B) Fractions enriched in specific granules (β1) and gelatinase granules (β2) from two different gradients were pooled and concentrated by centrifugation, and 1.5 × 107 cell equivalents were further analyzed by SDS-PAGE and immunoblotting with anti-FPRL1 antibody.
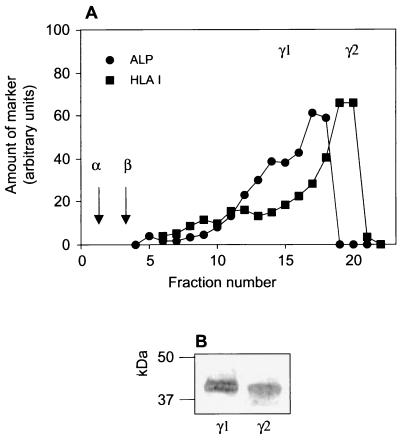
No trace of FPRL1 was seen in the upper part of the two-step gradient (Fig. 4), suggesting that FPRL1 is present in very small amounts in secretory vesicles and plasma membranes. Nonetheless, we isolated these organelles by flotation gradient centrifugation (15). As determined from the distribution profiles for latent alkaline phosphatase (a secretory vesicle marker) and HLA class I (a plasma membrane marker), the γ1 band contained the secretory vesicles, while the γ2 band contained the plasma membranes (Fig. 6A). The peak fractions containing the organelles were pooled separately, and the membranes were concentrated by centrifugation. When these concentrated samples (9 × 107 cell equivalents) were immunoblotted with the anti-FPRL1 antibody, we found detectable amounts of FPRL1 (Fig. 6B). In comparison, the secretory vesicles appeared to contain slightly more FPRL1 than the plasma membranes.
FIG. 6.
Presence of FPRL1 in secretory vesicles and plasma membranes. A postnuclear supernatant of disrupted neutrophils was fractionated on a flotation Percoll gradient. (A) Fractions were analyzed for latent alkaline phosphatase (ALP) (marker for secretory vesicles) and HLA class I antigen (marker for plasma membranes). The positions in the gradient of the azurophil granules (α) and the specific granules (β) are indicated by arrows. (B) Fractions enriched in secretory vesicles (γ1) and plasma membranes (γ2) from four different gradients were pooled and concentrated by centrifugation, and 9 × 107 cell equivalents were analyzed by SDS-PAGE and immunoblotting with anti-FPRL1 antibody.
Changes in the subcellular localization of FPRL1 upon LPS-induced priming.
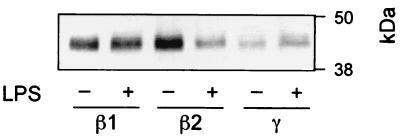
We finally examined the LPS-induced effect on the subcellular localization of FPRL1 by performing fractionations on three-step Percoll density gradients of two cell populations, one resting and one primed by LPS, with our standard protocol (see Materials and Methods). From these fractionations, we pooled separately the fractions corresponding to the specific granules (β1 fractions), the gelatinase granules (β2 fractions), and the plasma membranes and secretory vesicles (γ fractions). The relative positions of these fractions were determined by marker analyses (data not shown), and the pooled fractions were immunoblotted with the anti-FPRL1 antibody. As expected, the β1 fractions from the different cell populations contained the same amounts of FPRL1, while the β2 fraction from LPS-primed cells contained less FPRL1 than the β2 fraction from resting cells (Fig. 7). Concomitant with the decrease in the amount of FPRL1 in the β2 fraction upon LPS-induced priming, there was an increase in the amount of the receptor in the γ fraction, corresponding to the plasma membranes and secretory vesicles (Fig. 7). The latter result indicated that LPS-induced priming indeed mobilized the gelatinase granules and thereby FPRL1 to the cell surface.
FIG. 7.
LPS-induced changes in the subcellular localization of FPRL1. Postnuclear supernatants of disrupted neutrophils from a resting population (−) and one preincubated at 37°C for 30 min in the presence of LPS (+) were fractionated on three-step Percoll density gradients. Fractions enriched in specific granules (β1), gelatinase granules (β2), and plasma membranes and secretory vesicles (γ) from two different gradients were pooled and concentrated by centrifugation, and 1.2 × 107 cell equivalents were further analyzed by SDS-PAGE and immunoblotting with anti-FPRL1 antibody.
DISCUSSION
We show that LPS primes the neutrophil response to the H. pylori cecropin-like peptide Hp(2-20) and that FPRL1, the receptor for Hp(2-20), is stored in mobilizable neutrophil granules. We thus suggest that degranulation is a major mechanism for the LPS-induced priming of the Hp(2-20) response. Our results raise two questions. First, how do the data relate to previously proposed mechanisms for neutrophil priming by LPS and other agents? This is a question that we have begun to address. Second, what role does Hp(2-20) play in relation to the pathophysiology of H. pylori infection and subsequent inflammation? This is a question that we at present only can speculate upon.
The cellular responses induced by Hp(2-20) are in many ways similar to those induced by the well-known chemoattractant fMLF and, accordingly, the receptor engaged by Hp(2-20) possesses many similarities with FPR. The Hp(2-20) receptor, FPRL1, has 69% sequence identity with the FPR (48), and the two receptors are particularly similar in the transmembrane domains and the intracellular loops responsible for receptor signaling. The differences in the extracellular domains imply that the two receptors bind and are activated by different ligands. FPRL1 is a promiscuous receptor that, in addition to Hp(2-20) (11), also binds at least six unrelated peptides, proteins, or lipids (12, 13, 16, 20, 22, 34, 42, 43). In addition to triggering similar responses and sharing similar structures, FPR and FPRL1 have very similar subcellular localizations, as shown here. Fractionation studies revealed that neutrophils store their FPRL1 in mobilizable granules or vesicles. The major portion was localized in the gelatinase and specific granules, with a somewhat higher content in the gelatinase granules. The light membrane fractions had to be concentrated in order to obtain detectable amounts of FPRL1. In these fractions, FPRL1 was equally distributed between the plasma membranes and the secretory vesicles. The subcellular localization of FPRL1 is thus very similar to that described for FPR (41) in that both receptors are stored in the neutrophil compartments most easily mobilized in response to inflammatory stimuli, i.e., secretory vesicles and gelatinase granules.
The molecular mechanisms behind LPS-dependent priming of the response to other stimuli, including the chemoattractant fMLF (the most widely studied agonist in this context), have been extensively discussed. The suggested mechanisms include alterations of intracellular signaling pathways (increased protein phosphorylation, phospholipase activity, intracellular Ca2+ changes, and cross talk between increased Ca2+ levels and tyrosine phosphorylation), altered assembly of NADPH oxidase, and proteolytic processing of cell surface proteins (21, 27, 28, 44, 45, 47). In addition, there are studies reporting increased amounts of fMLF receptors (FPR) on LPS-primed neutrophils (2, 36, 46). This mechanism has not been regarded as a major mechanism behind the induction of the primed response, most probably due to the impact on the field by a study published in 1984 by Guthrie et al. (27) reporting that LPS-induced priming of the oxidative response was not accompanied by increased amounts of cell surface FPR. At that time, FPR was believed to be mobilized from a storage pool identified as specific granules. However, since then, secretory vesicles and gelatinase granules have been identified (7, 31); both of these store FPR (41) as well as FPRL1 (this study).
Based upon our finding that LPS-induced priming is accompanied by mobilization of the receptor-storing secretory vesicles and gelatinase granules (without much mobilization of specific granules), we challenge the prevailing view and suggest that receptor upregulation from granule stores indeed makes an important contribution to LPS-induced priming of neutrophil responses to chemoattractants such as Hp(2-20). This conclusion is supported by our results obtained with the nonprimable cytoplasts which, devoid of intracellular organelles (40), lack the ability to upregulate stored receptors. It is not possible to make a direct comparison between normal neutrophils and cytoplasts with regard to the magnitude of the activation, since the preparation of cytoplasts results in some loss of plasma membranes as well as some granule secretion (40). Our data, however, clearly show that LPS has no effect on their responsiveness to stimulation with Hp(2-20), suggesting a role for the granules in the priming process. Furthermore, our fractionation data show that the FPRL1 content is decreased in β2 fractions but increased in γ fractions upon LPS treatment, indicating that FPRL1 in gelatinase granules is mobilized to plasma membranes.
Since LPS treatment did not affect the NADPH oxidase response to the protein kinase C agonist phorbol myristate acetate, we conclude that direct effects on NADPH oxidase assembly or activity are not plausible explanations of the priming effect seen here. This conclusion was further strengthened by the cytoplast experiments, as the cytoplasts contained intact NADPH oxidase but still could not be primed by LPS.
The persistence of H. pylori in the mucosal lining has been suggested to be facilitated by antibacterial cecropin-like peptides generated by the microbe. This property gives H. pylori a competitive advantage over other microorganisms, as H. pylori organisms themselves are resistant to their own bactericidal compounds (39). The inflammatory response associated with H. pylori infection is probably also beneficial for the bacteria, by promoting the release of nutrients from the epithelial lining, enabling continued bacterial growth and persistence in the mucosal tissue (6). It should, however, be pointed out that an infected and inflamed gastric mucosa contains a large number of inflammatory mediators as well as bacterial products that influence both the survival of the invading microbe and the destruction of the tissue. A neutrophil-activating and bactericidal peptide such as Hp(2-20) may be one of the constituents in an H. pylori infection, as this peptide possesses several important functional characteristics; it is bactericidal, it is a neutrophil chemoattractant, and it activates phagocyte NADPH oxidase to produce reactive oxygen species (11). In addition, we can now add Hp(2-20) to the array of agonists for which LPS has important regulatory functions. LPS treatment simultaneously induces a primed state in neutrophils and mobilizes the secretory vesicles and the gelatinase granules. These organelles contain a substantial amount of stored FPRL1, suggesting that the mechanism behind LPS-induced priming of the response to Hp(2-20) lies at the level of granule (receptor) mobilization.
Acknowledgments
The skillful technical assistance of Maria Hjulström, Marie Samuelsson, and Pia Andersson is gratefully acknowledged.
The Swedish group was supported by the Swedish Society for Rheumatological Research, the Swedish Society for Medicine, the Swedish Medical Research Council, and the Swedish Network and Graduate School for Infection and Vaccinology. The work of the French group was supported by grants from the Commissariat à l'Energie Atomique and the Centre National de la Recherche Scientifique (CNRS).
Editor: R. N. Moore
REFERENCES
- 1.Allen, L. A. 2000. Modulating phagocyte activation: the pros and cons of Helicobacter pylori virulence factors. J. Exp. Med. 191:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almkvist, J., J. Fäldt, C. Dahlgren, H. Leffler, and A. Karlsson. 2001. Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl-Leu-Phe. Infect. Immun. 69:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, T., C. Dahlgren, P. D. Lew, and O. Stendahl. 1987. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in the cytosolic free Ca2+ level and action of phorbol myristate acetate. J. Clin. Investig. 79:1226-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 5.Bjerrum, O. W., and N. Borregaard. 1990. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand. J. Immunol. 31:305-313. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1993. Helicobacter pylori: microbiology of a ‘slow’ bacterial infection. Trends. Microbiol. 1:255-260. [DOI] [PubMed] [Google Scholar]
- 7.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 8.Borregaard, N., J. M. Heiple, E. R. Simons, and R. A. Clark. 1983. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 97:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 10.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 11.Bylund, J., T. Christophe, F. Boulay, T. Nyström, A. Karlsson, and C. Dahlgren. 2001. Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob. Agents Chemother. 45:1700-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang, N., I. M. Fierro, K. Gronert, and C. N. Serhan. 2000. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 191:1197-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christophe, T., A. Karlsson, C. Dugave, M. J. Rabiet, F. Boulay, and C. Dahlgren. 2001. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/LXA4R and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J. Biol. Chem. 276:21585-21593. [DOI] [PubMed] [Google Scholar]
- 14.Condliffe, A. M., E. Kitchen, and E. R. Chilvers. 1998. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin. Sci. (London). 94:461-471. [DOI] [PubMed] [Google Scholar]
- 15.Dahlgren, C., S. R. Carlsson, A. Karlsson, H. Lundqvist, and C. Sjölin. 1995. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem. J. 311:667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlgren, C., T. Christophe, F. Boulay, P. N. Madianos, M. J. Rabiet, and A. Karlsson. 2000. The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A(4) receptor. Blood 95:1810-1818. [PubMed] [Google Scholar]
- 17.Dahlgren, C., A. Johansson, H. Lundqvist, O. W. Bjerrum, and N. Borregaard. 1992. Activation of the oxygen-radical-generating system in granules of intact human neutrophils by a calcium ionophore (ionomycin). Biochim. Biophys. Acta 1137:182-188. [DOI] [PubMed] [Google Scholar]
- 18.Dahlgren, C., A. Johansson, and K. Orselius. 1989. Difference in hydrogen peroxide release between human neutrophils and neutrophil cytoplasts following calcium ionophore activation. A role of the subcellular granule in activation of the NADPH-oxidase in human neutrophils? Biochim. Biophys. Acta 1010:41-48. [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren, C., and A. Karlsson. 1999. Respiratory burst in human neutrophils. J. Immunol. Methods 232:3-14. [DOI] [PubMed] [Google Scholar]
- 20.De, Y., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeo, F. R., J. Renee, S. McCormick, M. Nakamura, M. Apicella, J. P. Weiss, and W. M. Nauseef. 1998. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Investig. 101:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng, X., H. Ueda, S. B. Su, W. Gong, N. M. Dunlop, J. L. Gao, P. M. Murphy, and J. M. Wang. 1999. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood 94:1165-1173. [PubMed] [Google Scholar]
- 23.Downey, G. P., T. Fukushima, L. Fialkow, and T. K. Waddell. 1995. Intracellular signaling in neutrophil priming and activation. Semin. Cell Biol. 6:345-356. [DOI] [PubMed] [Google Scholar]
- 24.English, D., and T. G. Gabig. 1986. Differentiation of cellular processes involved in the induction and maintenance of stimulated neutrophil adherence. Blood 67:1314-1322. [PubMed] [Google Scholar]
- 25.Follin, P., G. Briheim, and C. Dahlgren. 1991. Mechanisms in neutrophil priming: characterization of the oxidative response induced by formylmethionyl-leucyl-phenylalanine in human exudated cells. Scand. J. Immunol. 34:317-322. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb, C., K. Lau, L. R. Wasserman, and V. Herbert. 1965. Rapid charcoal assay for intrinsic factor (IF), gastric juice unsaturated B12 binding capacity, antibody to IF, and serum unsaturated B12 binding capacity. J. Hematol. 25:875-883. [PubMed] [Google Scholar]
- 27.Guthrie, L. A., L. C. McPhail, P. M. Henson, and R. B. Johnston, Jr. 1984. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharides. J. Exp. Med. 160:1656-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallett, M. B., and D. Lloyds. 1995. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol. Today 16:264-268. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson, A., P. Follin, H. Leffler, and C. Dahlgren. 1998. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 91:3430-3438. [PubMed] [Google Scholar]
- 30.Kjeldsen, L., O. W. Bjerrum, D. Hovgaard, A. H. Johnsen, M. Sehested, and N. Borregaard. 1992. Human neutrophil gelatinase: a marker for circulating blood neutrophils. Purification and quantitation by enzyme linked immunosorbent assay. Eur. J. Haematol. 49:180-191. [DOI] [PubMed] [Google Scholar]
- 31.Kjeldsen, L., H. Sengeløv, K. Lollike, N. M. H., and N. Borregaard. 1994. Isolation and characterization of gelatinase granules from human neutrophils. Blood 83:1640-1649. [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lang, J., F. Boulay, G. Li, and C. B. Wollheim. 1993. Conserved transducer coupling but different effector linkage upon expression of the myeloid fMet-Leu-Phe receptor in insulin secreting cells. EMBO J. 12:2671-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le, Y., H. Yazawa, W. Gong, Z. Yu, V. J. Ferrans, P. M. Murphy, and J. M. Wang. 2001. The neurotoxic prion peptide fragment PrP(106-126) is a chemotactic agonist for the G protein-coupled receptor formyl peptide receptor-like 1. J. Immunol. 166:1448-1451. [DOI] [PubMed] [Google Scholar]
- 35.Lundahl, J., C. Dahlgren, A. Eklund, J. Hed, R. Hernbrand, and G. Tornling. 1993. Quartz selectively down-regulates CR1 on activated human granulocytes. J. Leukoc. Biol. 53:99-103. [DOI] [PubMed] [Google Scholar]
- 36.McLeish, K. R., J. B. Klein, E. D. Lederer, K. Z. Head, and R. A. Ward. 1996. Azothemia, TNF alpha, and LPS prime the neutrophil oxidative burst by distinct mechanisms. Kidney Int. 50:407-416. [DOI] [PubMed] [Google Scholar]
- 37.Murphy, P. M., T. Ozcelik, R. T. Kenney, H. L. Tiffany, D. McDermott, and U. Francke. 1992. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 267:7637-7643. [PubMed] [Google Scholar]
- 38.Nathan, C. F. 1987. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J. Clin. Investig. 80:1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pütsep, K., C. I. Bränden, H. G. Boman, and S. Normark. 1999. Antibacterial peptide from H. pylori. Nature 398:671-672. [DOI] [PubMed] [Google Scholar]
- 40.Roos, D., A. A. Voetman, and L. J. Meerhof. 1983. Functional activity of enucleated human polymorphonuclear leukocytes. J. Cell Biol. 97:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sengeløv, H., F. Boulay, L. Kjeldsen, and N. Borregaard. 1994. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem. J. 299:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su, S. B., J. Gao, W. Gong, N. M. Dunlop, P. M. Murphy, J. J. Oppenheim, and J. M. Wang. 1999. T21/DP107, A synthetic leucine zipper-like domain of the HIV-1 envelope gp41, attracts and activates human phagocytes by using G-protein-coupled formyl peptide receptors. J. Immunol. 162:5924-5930. [PubMed] [Google Scholar]
- 43.Su, S. B., W. Gong, J. L. Gao, W. Shen, P. M. Murphy, J. J. Oppenheim, and J. M. Wang. 1999. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 189:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surette, M. E., N. Dallaire, N. Jean, S. Picard, and P. Borgeat. 1998. Mechanisms of the priming effect of lipopolysaccharides on the biosynthesis of leukotriene B4 in chemotactic peptide-stimulated human neutrophils. FASEB J. 12:1521-1531. [DOI] [PubMed] [Google Scholar]
- 45.Tauber, A. I., A. B. Karnad, K. L. Hartshorn, J. B. Myers, and J. H. Schwartz. 1989. Parameters of neutrophil activation: models of priming and deactivation, p. 297-309. In A. I. Tauber, B. U. Wintroub, and A. Stolper-Simon (ed.), Biochemistry of the acute allergic reactions: Fifth International Symposium. Alan R. Liss, Inc., New York, N.Y. [PubMed]
- 46.Vosbeck, K., P. Tobias, H. Mueller, R. A. Allen, K. E. Arfors, R. J. Ulevitch, and L. A. Sklar. 1990. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J. Leukoc. Biol. 47:97-104. [DOI] [PubMed] [Google Scholar]
- 47.Watson, F., and S. W. Edwards. 1998. Stimulation of primed neutrophils by soluble immune complexes: priming leads to enhanced intracellular Ca2+ elevations, activation of phospholipase D, and activation of the NADPH oxidase. Biochem. Biophys. Res. Commun. 29:819-826. [DOI] [PubMed] [Google Scholar]
- 48.Ye, R. D., and F. Boulay. 1997. Structure and function of leukocyte chemoattractant receptors. Adv. Pharmacol. 39:221-289. [DOI] [PubMed] [Google Scholar]
- 49.Ye, R. D., S. L. Cavanagh, O. Quehenberger, E. R. Prossnitz, and C. G. Cochrane. 1992. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 184:582-589. [DOI] [PubMed] [Google Scholar]