Abstract
The development of T1-cell-mediated immunity is required to clear a pulmonary Cryptococcus neoformans infection. The objective of these studies was to determine the mechanism by which tumor necrosis factor alpha (TNF-α) augments the development of pulmonary T1 immunity to C. neoformans infection. TNF-α expression was detected in lavage sample cells at days 2, 3, and 7 following C. neoformans infection. The numbers of CFU in the lung were not different between control and anti-TNF-α-treated mice at any time point examined during the afferent phase of the response (days 0 to 7). However, neutralization of TNF-α prevented the initiation of pulmonary clearance during the efferent phase of the response (day 14). Administration of anti-TNF-α monoclonal antibody (day 0) diminished the lung levels of TNF-α, interleukin-12 (IL-12), and gamma interferon (IFN-γ) induced by C. neoformans at day 7 postinfection. Neutralization of TNF-α (day 0) also altered the IFN-γ/IL-4 ratio in the lung-associated lymph nodes at day 7 following C. neoformans infection. Anti-TNF-α-treated mice developed a pulmonary eosinophilia at day 14 postinfection. Consistent with the pulmonary eosinophilia, anti-TNF-α-treated mice exhibited elevated serum immunoglobulin E and inhibition of the anticryptococcal delayed-type hypersensitivity response, indicating a shift toward a T2 response. Neutralization of IL-12 also prevented lung leukocyte production of IFN-γ in response to the infection. These findings demonstrate that afferent-phase TNF-α production is essential for the induction of IL-12 and IFN-γ and neutralization of early TNF-α results in a T2 shift of the T1/T2 balance of antifungal immunity.
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that has been increasingly recognized for its role in antigen-specific cell-mediated immune responses (33). Lipopolysaccharide and phagocytosis of microbes (20) are potent stimuli for TNF-α production by macrophages. TNF-α is an effector-phase molecule in innate immunity against acute bacterial infection and in acquired immunity against parasitic, protozoan, and fungal infection (20, 33). TNF-α is one of the first cytokines produced by macrophages following activation, and there is increasing evidence that TNF-α plays an important role in the afferent phase of acquired immunity.
TNF-α promotes dendritic cell migration from tissues into lymph nodes, can induce chemokines that would be important in the recruitment of antigen-presenting cells, and upregulates antigen presentation (20, 33). TNF-α is required for host defense against the fungi Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, Candida albicans, Pneumocystis carinii, Aspergillus fumigatus, and Paracoccidioides immitis (1, 4, 16, 23, 24, 32). The importance of TNF-α is illustrated by the observation that a single dose of anti-TNF-α serum at the onset of C. neoformans infection renders the mice unable to clear the infection even 5 weeks after treatment (16). While the importance of TNF-α in the development of antifungal immunity has been documented, it remains to be determined how afferent-phase TNF-α production drives the development of protective T1-type cell-mediated immunity to a fungus such as C. neoformans.
There are data indicating that the timing of TNF-α induction is critical (15, 16), and delayed induction (via virulence factor action or host physiologic factors) leads to permanent changes in cryptococcal clearance. The lungs are the portal of entry for C. neoformans, but innate immune defense mechanisms in the lungs are insufficient to control a C. neoformans infection. In contrast, depletion of CD4 and/or CD8 T cells impairs pulmonary clearance of C. neoformans (10, 14, 25). Thus, our objective was to determine the mechanism by which early TNF-α production (innate immunity) augments the development of adaptive T1-cell-mediated immunity.
MATERIALS AND METHODS
Mice.
Female CBA/J mice (weight, 16 ± 2 g) were obtained from the Jackson Laboratories (Bar Harbor, Maine). Mice were housed under pathogen-free conditions in enclosed filter-topped cages. Clean food and water were given ad libitum. The mice were handled and maintained using microisolator techniques, with daily veterinarian monitoring. Bedding from the mice was transferred weekly to cages of uninfected sentinel mice that were subsequently bled at weekly intervals and found to be negative for antibodies to mouse hepatitis virus, Sendai virus, and Mycoplasma pulmonis.
C. neoformans.
C. neoformans strain 52D was obtained from the American Type Culture Collection (24067). For infection, yeasts were grown to stationary phase (48 to 72 h) at 37°C in Sabouraud dextrose broth (1% Neopeptone and 2% dextrose; Difco, Detroit, Mich.) on a shaker. The cultures were then washed in nonpyrogenic saline, counted on a hemacytometer, and diluted to 3.3 × 105 CFU/ml in sterile nonpyrogenic saline.
Intratracheal inoculation of C. neoformans.
Mice were inoculated as previously described (16). Briefly, a 30-gauge needle (Becton Dickinson, Rutherford, N.J.) was bent and attached to a tuberculin syringe (Monoject, St. Louis, Mo.) filled with the diluted C. neoformans culture. The needle was inserted into the trachea of anesthetized mice, and 30 μl of inoculum was dispensed into the lungs (104 CFU). Aliquots of the inoculum were collected periodically to monitor the number of CFU delivered.
In vivo neutralization of TNF-α and IL-12.
C. neoformans-infected mice were given a single intraperitoneal injection of 0.25 mg of anti-TNF-α monoclonal antibody (MP6-XT3) at the time of infection. For the anti-interleukin-12 (IL-12) experiment, C. neoformans-infected mice were given 0.25 mg of anti-IL-12 monoclonal antibody (C17.15) by intraperitoneal injection on days 0, 3, and 6 of infection.
CFU assay.
For lung CFU, small aliquots were collected from lung digests. Ten-microliter aliquots of the lungs were plated out on Sabouraud dextrose agar (Difco, Detroit, Mich.) plates in duplicate 10-fold dilutions and incubated at room temperature. C. neoformans colonies were counted 2 to 3 days later, and the number of CFU was calculated.
BAL samples.
Following euthanasia, mice were lavaged by cannulation of the trachea with polyethylene tubing (PE50) attached to a 25-gauge needle on a tuberculin syringe. The lungs were lavaged twice with 0.75 ml of phosphate-buffered saline (PBS). The recovered fluid (1.3 to 1.4 ml total) was spun at 1,500 rpm, and the supernatant was removed and stored at −20°C for further analysis. Levels of IL-12 and TNF-α in the bronchoalveolar lavage (BAL) fluid were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using the manufacturer's instructions supplied with the cytokine-specific kits (OptEIA; PharMingen, San Diego, Calif.).
RT-PCR.
For reverse transcription (RT)-PCR, total RNA was isolated from lavage cells or lymph nodes using Trizol reagent (Life Technologies, Gaithersburg, Md.) as outlined in the Trizol protocol. The primer sequences for TNF-α were 5′-AGCACAGAAAGCATGATCCGCG-3′ (sense) and 5′-GACTTTCTCCTGGTATGAGATAGC-3′ (antisense). The primer sequences for IL-4 were 5′-GGAGCCATATCCACGGATGCGAC-3′ (sense) and 5′-GAATCCAGGCATCGAAAAGCCCG-3′ (antisense). The primer sequences for IFN-γ were 5′-GGCTGTTTCTGGCTGTTACTGCCACG-3′ (sense) and 5′-GACAATCTCTTCCCCACCCCGAATCAG-3′ (antisense). The primer sequences for β-actin were 5′-GTGGGGCGCCCCAGGCACCA-3′ (sense) and 5′-GCTGGCCGTGGTGGTGAAGC-3′ (antisense).
Preparation of lung leukocytes.
Lung leukocytes were isolated as previously described (16, 29). Briefly, the lungs were excised, minced, and enzymatically digested for 30 min using 15 ml of digestion buffer (RPMI, 5% fetal calf serum, antibiotics, and 1 mg/ml collagenase [Boehringer Mannheim]) per lung. Lung leukocytes were counted on a hemacytometer with trypan blue.
Cell differentials (neutrophils, macrophages, lymphocytes, and eosinophils) were visually counted from Wright-Giemsa-stained samples of lung cell suspensions cytospun onto glass slides (Shandon Cytospin, Pittsburgh, Pa.). The absolute number of a leukocyte subset was equal to the percentage of that cell subset multiplied by the total number of leukocytes in the lungs.
DTH assay.
Mice were tested for the development of delayed-type hypersensitivity (DTH) mediating T-cell immunity using a modification of a previously described footpad DTH assay (27). In brief, C. neoformans filtrate antigen (20 μl) was injected into the hind right footpad, and the hind left footpad was injected with 20 μl of dialyzed asparagine broth-2% bovine serum albumin (BSA). After 48 h, the thickness of each footpad was measured using a micrometer. The swelling in the right footpad was determined by subtracting the measurement of the right footpad from the measurement of the left. Uninfected mice were also challenged as a negative control for the assay.
Lung leukocyte culture, cytokine ELISA, and IgE ELISA.
Isolated leukocytes (from enzymatic digests) from individual mice were standardized to 15 × 106 cells/3 ml and cultured in complete medium without additional stimulation at 37°C and 5% CO2. Supernatants were harvested at 24 h and assayed for IFN-γ production by sandwich ELISA (OptEIA; Pharmingen, San Diego, Calif.). Peripheral venous blood was collected from individual mice, and serum was isolated and stored at −20°C until assayed for immunoglobulin E (IgE) using a murine IgE ELISA kit (Pharmingen).
Statistics.
The mean ± standard error (SE) was determined for each treatment group in the individual experiments. Statistical significance was calculated using a t test, with a significance level of P < 0.05 for a single comparison.
RESULTS
Induction of TNF-α early in the course of pulmonary C. neoformans infection.
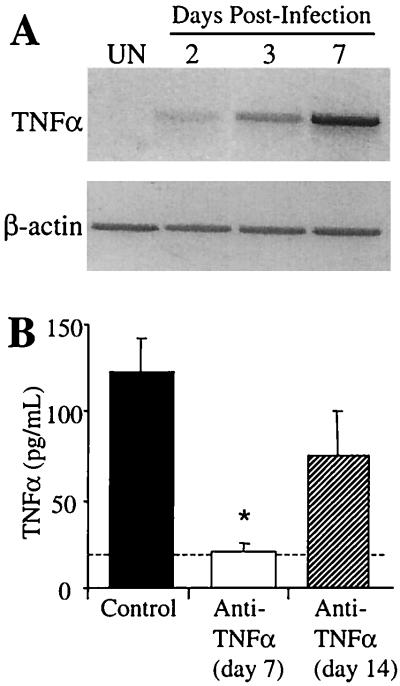
The first objective of these studies was to determine the kinetics of TNF-α expression during the first week of a C. neoformans infection. CBA/J mice were inoculated intratracheally with 104 CFU of C. neoformans, and TNF-α expression was examined in bronchoalveolar lavage cells isolated at days 2, 3, and 7 postinfection. Analysis of the cell types present in the lavage fluid of C. neoformans-infected animals demonstrated that >90% were macrophages at days 2 and 3, whereas at day 7 there were approximately 75% macrophages and 25% neutrophils (data not shown). TNF-α RNA was barely detectable at day 2 of infection but was clearly visible by day 3 (Fig. 1A). At day 7, TNF-α expression was strongly expressed by the lavage cells of C. neoformans-infected mice. These findings demonstrate that alveolar macrophages are likely the major source of early TNF-α following C. neoformans infection.
FIG. 1.
TNF-α expression in C. neoformans-infected mice. (A) TNF-α expression was analyzed in lavage cells of C. neoformans-infected mice at days 2, 3, and 7 by RT-PCR. β-Actin expression demonstrates equal loading of RNA among all samples. UN, uninfected mice. (B) BAL fluid was collected from C. neoformans-infected control and anti-TNF-α-treated (day 0) mice at days 7 and 14 postinfection. TNF-α levels were determined by ELISA. Results are expressed as the mean ± SE for eight mice per treatment group from two separate experiments. ∗, P < 0.05 compared with control C. neoformans-infected mice. The dashed line represents cytokine levels in uninfected animals.
Effect of TNF-α neutralization on the pulmonary burden of C. neoformans.
The pulmonary burden of C. neoformans was examined to determine whether neutralization of early TNF-α would increase the number of cryptococci in the lungs during the course of infection. Mice were infected intratracheally with C. neoformans, and anti-TNF-α monoclonal antibody was administered by a single intraperitoneal injection at the time of infection (day 0). TNF-α protein was detected in the lavage fluid of control infected mice at day 7 postinfection. Treatment with anti-TNF-α monoclonal antibody at the time of infection significantly diminished the levels of TNF-α induced by C. neoformans through day 7 of infection (Fig. 1B). However, TNF-α levels increased between days 7 and 14, indicating that the neutralization of TNF-α was only transient (lasting between 7 and 14 days).
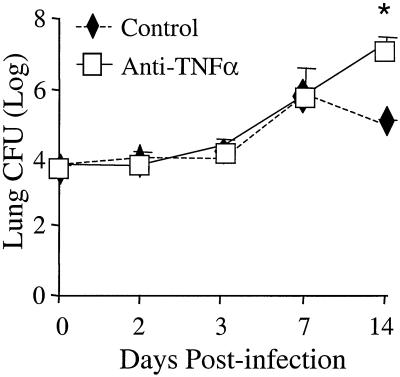
The cryptococcal burden in the lungs of control mice increased over 1,000-fold from days 0 to 7 and began to decrease by day 14 postinfection (Fig. 2). Lung CFU were not significantly different between control and anti-TNF-α-treated mice on days 2, 3, and 7 postinfection. However, lung CFU continued to increase in anti-TNF-α-treated mice through day 14, indicating impaired clearance in these animals (Fig. 2). These findings demonstrate that blocking afferent-phase TNF-α does not alter growth of C. neoformans prior to the development of adaptive immunity (days 0 to 7) but does impair cryptococcal clearance at day 14 postinfection.
FIG. 2.
Lung CFU in C. neoformans-infected control and anti-TNF-α-treated mice. CBA/J mice were infected intratracheally with 10,000 CFU and treated with anti-TNF-α monoclonal antibody at day 0. Results are expressed as the mean CFU ± SE for eight mice per group per time point from two separate experiments. ∗, P < 0.05 compared with C. neoformans-infected mice.
Neutralization of TNF-α inhibits production of IL-12 and IFN-γ.
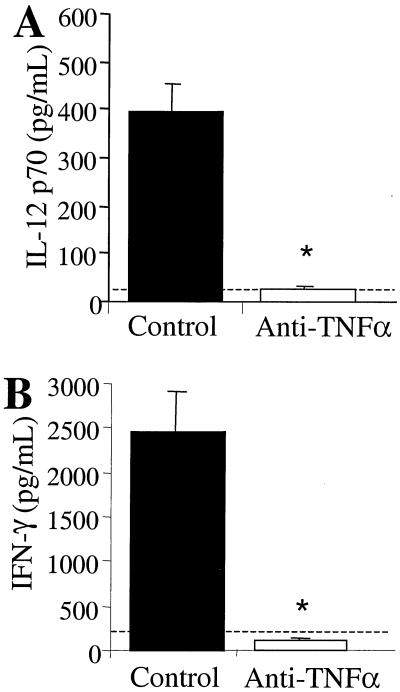
We next examined whether afferent-phase TNF-α neutralization could inhibit the expression of cytokines that drive T1 immunity to C. neoformans. To address this question, the levels of IL-12 in BAL fluid from control and anti-TNF-α-treated mice were analyzed by ELISA. No detectable levels of IL-12 were observed at days 2 and 3 postinfection (data not shown). IL-12 levels in the BAL fluid of control mice increased significantly by day 7 postinfection (Fig. 3A). Neutralization of TNF-α at the time of infection markedly inhibited IL-12 induction at day 7 (Fig. 3A).
FIG. 3.
IL-12 (A) and IFN-γ (B) levels in C. neoformans-infected control and anti-TNF-α-treated mice. (A) BAL fluid was collected from control and anti-TNF-α-treated (day 0) mice at day 7 postinfection. The levels of IL-12 p70 were determined by ELISA. (B) Leukocytes were isolated from whole lungs at day 7 postinfection and cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IFN-γ by ELISA. Results are expressed as the mean ± SE for eight mice per treatment group from two separate experiments. ∗, P < 0.05 compared with control C. neoformans-infected mice. The dashed line represents cytokine levels in uninfected animals.
IFN-γ production was assessed using leukocytes isolated from the lungs of control and anti-TNF-α-treated mice. Lung leukocytes were cultured for 24 h without additional stimulation, and culture supernatants were analyzed for IFN-γ by ELISA. At day 7, leukocytes from control animals produced substantial amounts of IFN-γ, whereas leukocytes from anti-TNF-α-treated mice produced very low levels of IFN-γ (Fig. 3B). Thus, afferent-phase TNF-α is required for the early induction of IL-12 and IFN-γ by lung leukocytes during a pulmonary C. neoformans infection.
Modulation of T1 and T2 responses by neutralization of afferent-phase TNF-α.
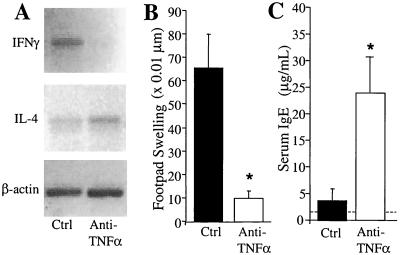
Neutralization of afferent-phase TNF-α alters IL-12 and IFN-γ production in the lungs during the first week of a C. neoformans infection (Fig. 3); therefore, we examined the T1/T2 cytokine profile in the lung-associated lymph nodes of control and anti-TNF-α-treated mice. As demonstrated in Fig. 4A, IFN-γ expression was induced in the lymph nodes of control mice but was undetectable in the nodes isolated from anti-TNF-α-treated animals at day 7 postinfection. IL-4 expression was detected in the lymph nodes of control mice; however, the induction of IL-4 was equal or greater in the anti-TNF-α-treated mice (Fig. 4A). Thus, neutralization of TNF-α during the afferent phase of the response to C. neoformans decreases the IFN-γ/IL-4 expression ratio in the lung-associated lymph nodes, which suggests a T1 to T2 switch.
FIG. 4.
Lack of a T1 response in anti-TNF-α-treated mice. (A) Cytokine expression in the lung-associated lymph nodes of C. neoformans-infected control (Ctrl) and anti-TNF-α-treated (day 0) mice. Lung-associated lymph nodes were removed at day 7 and pooled for RNA isolation (three mice per group). The expression of IFN-γ and IL-4 was analyzed by RT-PCR using 500 ng of node RNA. β-Actin expression is shown to demonstrate equal loading of RNA among all samples. Results are representative of three separate experiments. (B) Anticryptococcal DTH in C. neoformans-infected control and anti-TNF-α-treated mice. Infected mice in both groups were tested for DTH at day 35 using a footpad DTH assay described in the text. Uninfected mice did not have a significant footpad response to the C. neoformans antigen. (C) Serum IgE levels in C. neoformans-infected control and anti-TNF-α-treated mice. Total serum IgE was measured at day 21 by ELISA. The dashed line represents levels in uninfected animals. Results are expressed as the mean ± SE for eight mice per treatment group from two separate experiments. ∗, P < 0.05 compared to control mice.
In light of the cytokine expression in the lymph nodes and lungs, we next examined whether the immune response in anti-TNF-α-treated mice had shifted to a T2 response. Control mice exhibited a strong footpad DTH reaction following challenge with cryptococcal filtrate antigen. In contrast, anticryptococcal DTH responses were diminished in anti-TNF-α-treated mice (Fig. 4B). Control mice had low levels of serum IgE at day 21 postinfection; however, anti-TNF-α-treated mice had significantly elevated levels of serum IgE (Fig. 4C). IL-4 was also slightly elevated and IL-5 was significantly elevated in lung leukocyte cultures from anti-TNF-α-treated mice (data not shown).
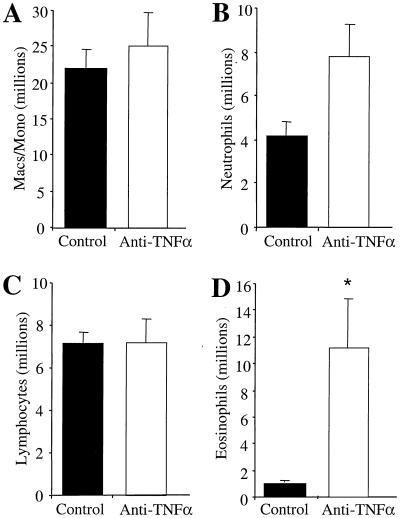
Lung leukocytes were also analyzed at day 14 postinfection to characterize cell populations affected by TNF-α neutralization. The most striking change was the increased recruitment of eosinophils into the lungs of anti-TNF-α-treated mice (Fig. 5D). Control mice had few eosinophils in their lungs, while anti-TNF-α-treated mice had >11 million eosinophils in their lungs at day 14 (Fig. 5D). The recruitment of macrophages, neutrophils, and lymphocytes was unaffected by anti-TNF-α (day 0) treatment (Fig. 5A to C). These data demonstrate that neutralization of afferent-phase TNF-α prevents the development of anticryptococcal DTH and results in elevated IgE levels and pulmonary eosinophilia, indicating a shift to a T2 response in anti-TNF-α-treated mice.
FIG. 5.
Analysis of leukocyte subsets in C. neoformans-infected control mice and anti-TNF-α-treated mice. Whole lungs from infected control and anti-TNF-α-treated (day 0) mice were removed and enzymatically digested to disperse lung leukocytes. Lung leukocyte differentials were determined from C. neoformans-infected and anti-TNF-α-treated mice at day 14 postinfection. Leukocytes isolated from the lungs were analyzed by Wright-Giemsa staining as described in the text. Results are expressed as the mean number per lung ± SE for eight mice per treatment group from two separate experiments. ∗, P < 0.05 compared with C. neoformans-infected mice.
Neutralization of IL-12 inhibits IFN-γ production.
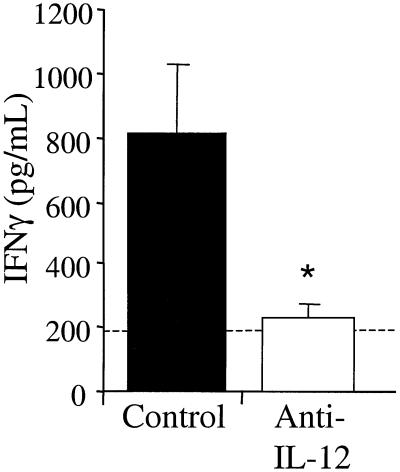
To determine the relationship between IL-12 and IFN-γ production during pulmonary C. neoformans infection, anti-IL-12 monoclonal antibody was administered by intraperitoneal injection on days 0, 3, and 6 of infection. Lung leukocytes were isolated at day 7 postinfection and cultured for 24 h without additional stimulation. As previously shown, leukocytes from control animals produced large amounts of IFN-γ at day 7 postinfection. However, neutralization of IL-12 blocked IFN-γ production by lung leukocytes at day 7 (Fig. 6). These data demonstrate that IL-12 is required for the early induction of IFN-γ during a pulmonary C. neoformans infection.
FIG. 6.
IFN-γ levels in C. neoformans-infected control and anti-IL-12-treated mice. CBA/J mice were infected intratracheally with 10,000 CFU and treated with anti-IL-12 monoclonal antibody at days 0, 3, and 6. Leukocytes were isolated from whole lungs at day 7 postinfection and cultured for 24 h without additional stimulation. Supernatants were collected and assayed for IFN-γ by ELISA. Results are expressed as the mean ± SE for eight mice per treatment group from two separate experiments. ∗, P < 0.05 compared with control C. neoformans-infected mice. The dashed line represents cytokine levels in uninfected animals.
DISCUSSION
These studies have identified a critical early window of TNF-α production that is required for the development of protective immunity against pulmonary C. neoformans infection. TNF-α is expressed during the first week of infection by alveolar macrophages via a T-cell-independent mechanism but does not enhance the fungicidal activity of the resident phagocytes in the lungs. Blocking afferent TNF-α induction inhibits the induction of IL-12 and IFN-γ in the lungs following C. neoformans infection, and blocking IL-12 also inhibits IFN-γ production. The end result of TNF-α neutralization at the time of infection is an altered IFN-γ/IL-4 ratio in the lung-associated lymph nodes, pulmonary eosinophilia, elevated serum IgE levels, and lack of C. neoformans-specific DTH reactivity. These features are all consistent with an upregulation of T2 immunity to the fungus. Thus, if TNF-α production is delayed, protective T1 immunity fails to develop in the lungs, and the T1/T2 balance shifts toward T2.
One of the major roles of afferent TNF-α production is the induction of IL-12 and IFN-γ in the lungs following C. neoformans infection (Fig. 3). Consistent with our current studies, we have previously demonstrated that infection with the highly virulent cryptococcal strain 145A does not induce (i) TNF-α or IFN-γ until later in the infection or (ii) protective T1 immunity (13, 29). In addition, Kawakami and colleagues have reported that infection with the highly virulent C. neoformans strain YC-11 does not induce TNF-α, IL-12, and IFN-γ (18, 19), but treatment of the mice with IL-12 stimulated IFN-γ production in the lungs and protected them against infection (19).
In addition to TNF-α, IL-12 was also required for IFN-γ induction in the lungs during C. neoformans infection (Fig. 6). TNF-α and IL-12 production by macrophages is also required for IFN-γ production during Listeria monocytogenes infection (36), while neutralization of TNF-α with a soluble TNF receptor fusion protein can inhibit Th1 polarization in vitro through the inhibition of IL-12 and IFN-γ (2). In contrast, a report by Rayhane et al. has shown that TNF-α is not required for induction of IFN-γ and IL-12 during C. neoformans infection (30). However, these differences are almost certainly due to different routes of infection (intravenous versus intratracheal), kinetics of cytokine analysis (day 16 versus day 7), and sites analyzed. Altogether, our studies indicate that TNF-α is a critical proximal signal required for the induction of IL-12 in the lungs, which then drives the production of IFN-γ to promote the development of protective T1-cell-mediated immunity to C. neoformans.
We have also demonstrated that the absence of afferent-phase TNF-α results in an upregulation of T2-type immunity to cryptococci, as characterized by increased IL-4/IFN-γ expression ratios in the pulmonary lymph nodes, eosinophil recruitment into the lungs, and elevated IgE levels in the serum. Our findings are consistent with a recent report that the lack of IFN-γ can increase airway eosinophilia (5). IFN-γ knockout or neutralization of either IFN-γ or IL-12 at the time of infection also increases the number of eosinophils in the lungs at day 14 of a C. neoformans infection (6, 12; T. Traynor, submitted for publication). IFN-γ knockout mice are also more susceptible to both pulmonary and intravenous C. neoformans infection (Traynor, submitted) (6, 40). Mice deficient in TNF-α also exhibit elevated levels of IL-4 and IL-10 in response to Candida albicans and Histoplasma capsulatum (1, 24). Thus, in the absence of early TNF-α induction, the T1/T2 balance of the immune response against C. neoformans shifts toward T2.
Alveolar macrophages can produce TNF-α in response to C. neoformans and are the first cells to come in contact with C. neoformans following pulmonary infection. These data are consistent with previous reports that human and murine alveolar macrophages are capable of producing significant amounts of TNF-α following exposure to C. neoformans (13, 21). TNF-α expression was not diminished in the lungs of T-cell-depleted animals, demonstrating that early TNF-α induction during C. neoformans infection does not require T cells (data not shown). However, other cells of innate immunity, such as neutrophils, NK cells, and γδ T cells, are also potential sources of TNF-α early in infection, and their roles remain to be determined.
Recent studies on the role of dendritic cells in induction of protective immunity to C. neoformans suggest that TNF-α plays an important role in dendritic cell biology (S. Bauman, G. B. Huffnagle, and J. Murphy, unpublished data). In T-cell receptor transgenic mice, TNF-α can serve as a cofactor with IL-1α in promoting in vitro differentiation of Th1 cells by dendritic cells (31), and TNF-α plays a role in dendritic cell trafficking into lymph nodes (20, 33). Thus, the ability of the host to produce appropriate early signal molecules such as TNF-α ultimately influences the development of cell-mediated immunity to C. neoformans.
In terms of microbial pathogenesis, it is intriguing that we cannot detect TNF-α expression at day 1 of an in vivo infection model. High levels of TNF-α are usually detected within hours following bacterial infection. Fungal cell walls (mannoproteins and β-glucans) are potent inducers of TNF-α (3, 7, 34, 35, 37). However, fungal virulence factors such as polysaccharide capsule and melanin in C. neoformans can downregulate TNF-α production by macrophages (13, 39). NK cell TNF-α production is also diminished following contact with cryptococci (26). In addition, cryptococcal polysaccharide capsule can induce IL-10 (a potent antagonist of TNF-α production) and induce TNF receptor shedding (8, 22, 38). The virulence factor W-1 of Blastomyces dermatitidis can also inhibit TNF-α production (9). Consistent with these observations is that highly virulent isolates of C. neoformans induce significantly less TNF-α following infection (13, 18, 29).
TNF-α is also required for protective host defense against the fungi Histoplasma capsulatum, Blastomyces dermatitidis, Candida albicans, Pneumocystis carinii, Aspergillus fumigatus, and Paracoccidioides immitis (1, 4, 23, 24, 32). Recently, we have identified that C. neoformans, C. albicans, H. capsulatum, B. dermatitidis, and A. fumigatus all produce prostaglandins E2 and D2 (28). Fungus-derived prostaglandin E2 can inhibit TNF-α and enhance IL-10 production by leukocytes (28). These findings indicate that production of virulence factors by fungi and their interaction with host leukocytes is a key determinant in the induction of TNF-α.
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute grants R01-065912 (to G.B.H.), R01-HL63670 (to G.B.H.), R01-HL51082 (to G.B.T.), and T32-HL07749 (to A.C.H.); Burroughs-Wellcome Fund (to G.B.H.); and Department of Veterans Affairs Merit Grant (to G.B.H).
Editor: R. N. Moore
REFERENCES
- 1.Allendoerfer, R., and G. J. Deepe. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. [PubMed] [Google Scholar]
- 2.Becher, B., M. Blain, P. S. Giacomini, and J. P. Antel. 1999. Inhibition of Th1 polarization by soluble TNF receptor is dependent on antigen-presenting cell-derived IL-12. J. Immunol. 162:684-688. [PubMed] [Google Scholar]
- 3.Chaka, W., A. F. Verheul, V. V. Vaishnav, R. Cherniak, J. Scharringa, J. Verhoef, H. Snippe, and I. M. Hoepelman. 1997. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect. Immun. 65:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn, L., C. Herrick, N. Niu, R. J. Homer, and K. Bottomly. 2001. IL-4 promotes airway eosinophilia by suppressing IFN-γ production: defining a novel role for IFN-γ in the regulation of allergic airway inflammation. J. Immunol. 166:2760-2767. [DOI] [PubMed] [Google Scholar]
- 6.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delfino, D., L. Cianci, M. Migliardo, G. Mancuso, V. Cusumano, C. Corradini, and G. Teti. 1996. Tumor necrosis factor-inducing activities of Cryptococcus neoformans components. Infect. Immun. 64:5199-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, Z. M., and J. Murphy. 1996. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J. Clin. Investig. 97:689-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel-Jimenez, B., M. Wuthrich, T. Brandhorst, and B. S. Klein. 2001. The WI-1 adhesin blocks phagocyte TNFα production, imparting pathogenicity on Blastomyces dermatitidis. J. Immunol. 166:2665-2673. [DOI] [PubMed] [Google Scholar]
- 10.Hill, J. O., and P. L. Dunn. 1993. A T-cell-independent protective host response against Cryptococcus neoformans expressed at the primary site of infection in the lung. Infect. Immun. 61:5302-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill, J. O., and A. G. Harmsen. 1991. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J. Exp. Med. 173:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant CB-17 mice. Am. J. Respir. Cell. Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 13.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Downregulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507-3516. [PubMed] [Google Scholar]
- 14.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., and L. K. McNeil. 1999. Dissemination of C. neoformans to the central nervous system: role of chemokines, Th1 immunity, and leukocyte recruitment. J. Neurovirol. 5:76-81. [DOI] [PubMed] [Google Scholar]
- 16.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNFα is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 17.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami, K., X. Qifeng, M. Tohyama, M. H. Qureshi, and A. Saito. 1996. Contribution of tumor necrosis factor-alpha (TNF-alpha) in host defense mechanism against Cryptococcus neoformans. Clin. Exp. Immunol. 106:468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami, K., M. Tohyama, X. Qifeng, and A. Saito. 1997. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of IL-12. Infect. Immun. 65:1307-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krakauer, T., J. Vilcek, and J. J. Oppenheim. 1999. Proinflammatory cytokines: TNF, and IL-1 families, chemokines, TGF-β, and others, p. 775-811. In W. E. Paul (ed.), Fundamental immunology. Lippincott-Raven, Philadelphia, Pa.
- 21.Levitz, S. M., A. Tabuni, H. Kornfeld, C. C. Reardon, and D. T. Golenbock. 1994. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect. Immun. 62:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitz, S. M., A. Tabuni, S. H. Nong, and D. T. Golenbock. 1996. Effects of interleukin-10 on human peripheral blood mononuclear cell responses to Cryptococcus neoformans, Candida albicans, and lipopolysaccharide. Infect. Immun. 64:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 24.Mencacci, A., E. Cenci, G. Del Sero, C. F. d'Ostiani, M. P., C. Montagnoli, A. Bacci, F. Bistoni, V. F. J. Quesniaux, B. Ryffel, and L. Romani. 1998. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-α double-deficient mice infected with Candida albicans. Int. Immunol. 10:37-48. [DOI] [PubMed] [Google Scholar]
- 25.Mody, C. H., G. H. Chen, C. Jackson, J. L. Curtis, and G. B. Toews. 1994. In vivo depletion of murine CD8-positive T cells impairs survival during infection with a highly virulent strain of Cryptococcus neoformans. Mycopathologia 125:7-17. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, J., A. Zhou, and S. C. Wong. 1997. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infect. Immun. 65:4564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, J. W., and N. Pahlavan. 1979. Cryptococcal culture filtrate antigen for detection of delayed-type hypersensitivity in cryptococcosis. Infect. Immun. 25:284.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, and G. B. Toews. 2001. MIP-1α/CCL3 regulatory effects on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rayhane, N., O. Lortholary, C. Fitting, J. Callebert, M. Huerre, F. Dromer, and J.-M. Cavaillon. 1999. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α-deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, interferon-γ, and interleukin-12. J. Infect. Dis. 180:1637-1647. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya, K., D. Robinson, F. Zonin, S. B. Hartley, S. E. Macatonia, C. Somoza, C. A. Hunter, K. M. Murphy, and O. G. Anne. 1998. IL-1α and TNFα are required for IL-12-induced development of Th1 cells producing high levels of IFN-γ in BALB/c but not C57BL/6 mice. J. Immunol. 160:1708-1716. [PubMed] [Google Scholar]
- 32.Souto, J. T., F. Figueiredo, A. Furlanetto, K. Pfeffer, M. A. Rossi, and J. S. Silva. 2000. Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am. J. Pathol. 156:1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson, A. W. 1998. The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.
- 34.Tokunaka, K., N. Ohno, Y. Adachi, S. Tanaka, H. Tamura, and T. Yadomae. 2000. Immunopharmacological and immunotoxicological activities of a water-soluble (1→3)-β-d-glucan, CSBG, from Candida spp. Int. J. Immunopharmacol. 22:383-394. [DOI] [PubMed] [Google Scholar]
- 35.Torosantucci, A., P. Chiani, and A. Cassone. 2000. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the beta-1,6 glucan of the fungal cell wall. J. Leukoc. Biol. 68:923-932. [PubMed] [Google Scholar]
- 36.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vecchiarelli, A., M. Puliti, A. Torosantucci, A. Cassone, and F. Bistoni. 1991. In vitro production of tumor necrosis factor by murine splenic macrophages with mannoprotein constituents of Candida albicans cell wall. Cell. Immunol. 134:65-76. [DOI] [PubMed] [Google Scholar]
- 38.Vecchiarelli, A., C. Retini, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect. Immun. 64:2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, C. Tascini, T. Beccari, and T. R. Kozel. 1995. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect. Immun. 63:2919-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 94:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]