Abstract
Salmonella enterica serovar Typhimurium is a facultative intracellular pathogen that inhabits a vacuolar compartment, called the Salmonella-containing vacuole (SCV), in infected host cells. Maintenance of the SCV is accomplished by SifA, and mutants of this Salmonella pathogenicity island 2 type III effector replicate more efficiently in epithelial cells. Here we demonstrate that enhanced replication of sifA mutants occurs in the cytosol of these cells. Increased replication of wild-type bacteria was also observed in cells treated with wortmannin or expressing Rab5 Q79L or Rab7 N125I, all of which caused a loss of SCV integrity. Our findings demonstrate the requirement of the host cell endosomal system for maintenance of the SCV and that loss of this compartment allows increased replication of serovar Typhimurium in the cytosol of epithelial cells.
Salmonella enterica serovar Typhimurium can cause a number of diseases, including gastroenteritis in humans and a systemic disease in mice that resembles typhoid fever (23, 41). Both in vitro (10) and in vivo (32, 33) evidence suggests that this pathogen inhabits a vacuolar compartment in infected cells, called the Salmonella-containing vacuole (SCV), during the course of these infections. Modulation of SCV interaction with the host endocytic system is thought to represent a major virulence strategy of serovar Typhimurium, a strategy shared by other intracellular pathogens (9). Principally implicated in altering SCV maturation are two different type III secretion systems (TTSS). The Salmonella pathogenicity island 1 (SPI-1)-encoded TTSS mediates invasion of nonphagocytic epithelial cells via a ruffling mechanism that is distinct from phagocytosis (6, 11). This uptake mechanism may dictate maturation of the SCV, since early events include the avoidance of lysosomes (13). A second TTSS, encoded on SPI-2, is induced inside cells and is essential for survival and replication in macrophages (28, 34). This system blocks delivery of the NADPH oxidase (12, 44, 45) in macrophages and may also play a role in avoiding lysosomes in this cell type (42).
Recent data have shown that the SPI-2 TTSS promotes integrity of the SCV through the actions of SifA, a translocated effector of this system (3, 5, 21). Deletion of sifA leads to loss of the vacuole surrounding intracellular bacteria at late times (10 h) following infection of murine macrophages in vitro. At this time, sifA mutants are predominantly located in the macrophage cytosol and are unable to replicate (3). Recent confocal analysis of the spleens of infected mice has confirmed a role for SifA in maintaining the SCV in macrophages in vivo (33). In epithelial cells, SifA mediates a unique phenotype characterized by extensive tubulation of the SCV, known as Salmonella-induced filaments (Sifs [16]). Deletion of sifA blocks Sif formation in epithelial cells (39). Surprisingly, the effect of this mutation on intracellular replication is the opposite of that in macrophages: sifA mutants replicate more efficiently than wild-type bacteria in epithelial cells, and the basis for this difference is unknown (39).
To examine this phenotype, we infected HeLa epithelial cells with serovar Typhimurium SL1344 for 10 h and examined the location of intracellular bacteria by confocal microscopy (5). As shown in Fig. 1A (upper panels), wild-type bacteria were localized within lysosomal-associated membrane protein 1 (LAMP-1+) vacuoles, and Sif formation was witnessed in approximately 50% of infected cells. This is consistent with previous studies demonstrating rapid LAMP-1 acquisition by the SCV following invasion and retention of this marker throughout the course of infection in this cell type (16, 26, 38). In a minority of wild-type infected cells (previously estimated to be 1 to 5% of the total number of intracellular bacteria under similar conditions [15]), intracellular bacteria lacking LAMP-1 were observed (Fig. 1, middle panels), indicating rupture of the SCV. In these cells, loss of LAMP-1 coincided with an increase in shed lipopolysaccharides (LPS) throughout the cytosol and a change in bacterial morphology. As shown, loss of the SCV led to a pronounced elongation of cytosolic bacteria when compared to those within vacuoles (Fig. 1).
FIG. 1.
Replication of serovar Typhimurium in the cytosol of HeLa epithelial cells. (A) HeLa cells were infected for 10 h with either wild-type or sifA serovar Typhimurium SL1344, as indicated, and then fixed with 2.5% paraformaldehyde. Cells were coimmunostained for LAMP-1 and Salmonella LPS, as indicated, and processed for confocal microscopy (5). Arrowheads indicate LAMP-1+ bacteria within Salmonella-containing vacuoles, while arrows indicate the elongated and swollen shape of LAMP-1− bacteria which are present in the cytosol and undergoing replication. (B) Detection of phoP expression. HeLa cells were infected by sifA serovar Typhimurium SL1344 with a plasmid encoding the destabilized mutant of GFP driven by the phoP promoter for 10 h, and then they were processed for confocal microscopy, as above. Arrowheads indicate LAMP-1+ bacteria expressing high levels of GFP, while arrows indicate LAMP-1− bacteria which are present in the cytosol and do not express GFP. Size bar, 10 μm.
To confirm that loss of LAMP-1 indicates rupture of the SCV and is not the result of altered SCV trafficking, we infected cells with bacteria expressing a destabilized mutant of green fluorescent protein (GFP) (Clontech) driven by the phoP promoter (to be described elsewhere). Consistent with previous reports, expression of phoP was induced in the SCV in response to low Mg2+ levels (estimated to be 10 to 50 μM [14]) in this compartment (17, 20), with 51% ± 10% (average ± standard deviation; n = 3) of LAMP-1+ wild-type bacteria expressing GFP. In contrast, only 11% ± 6% (n = 3) of LAMP-1− serovar Typhimurium expressed detectable amounts of GFP, indicative of high Mg2+ concentrations in the cytosol and downregulation of the phoP promoter. Similar results were seen with sifA mutants, although a higher percentage of LAMP-1− bacteria were observed (Fig. 1B). These findings demonstrate that loss of LAMP-1 coincides with disruption of the Salmonella-containing vacuole and release of the bacteria into the cytosol.
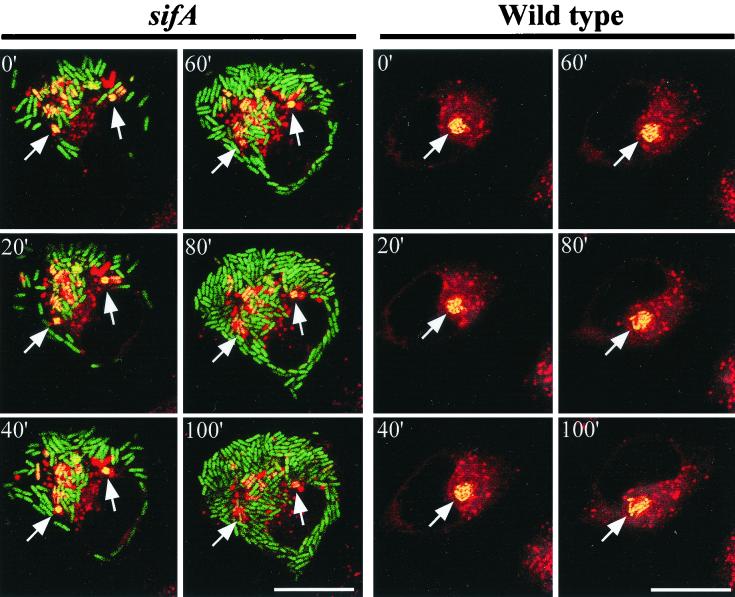
While cytosolic replication of wild-type serovar Typhimurium was observed, the majority of these bacteria were located within LAMP-1+ SCV and appeared to be undergoing extensive replication in this compartment (Fig. 1A, upper panels). In contrast, deletion of sifA appeared to block intravacuolar replication, as only small clusters (one to four bacteria per vacuole) of LAMP-1+ bacteria were observed (Fig. 1A, lower panels). Consistent with the results of Beuzón et al. (3), large numbers of LAMP-1− bacteria were observed in these cells, indicating that cytosolic replication of the sifA mutant was also occurring. To directly visualize replication of sifA mutants of serovar Typhimurium in HeLa cells, we performed time lapse confocal imaging of infected cells (Fig. 2). For these experiments, infected cells were loaded with Lysotracker DND-99 to label acidic compartments (in red), which include the SCV (1, 30). This provides a convenient method of determining which bacteria (identified by constitutive expression of GFP) are present in vacuoles or the cytosol. As shown in Fig. 2 (left-hand series), sifA mutants were observed to move freely in the cytosol and underwent rapid replication in this compartment. By contrast, sifA mutants present in acidified vacuoles (arrows) did not replicate over the 2-h imaging period. Wild-type bacteria were observed predominantly in acidified vacuoles (right-hand panels) and were capable of replicating in these compartments, albeit with a lower replication rate.
FIG. 2.
Time lapse confocal microscopy of serovar Typhimurium replication in infected epithelial cells. HeLa cells were grown in glass chambers (Nunc) and then infected for 6 h with either the wild type or sifA serovar Typhimurium SL1344 as indicated. The medium was then replaced with HEPES-buffered growth medium, and cells were loaded with 100 nM Lysotracker DND-99 (Molecular Probes) to label acidified compartments (in red). Chambers were mounted onto an inverted Zeiss microscope with a heated incubation chamber at 35 to 37°C, and time lapse images were acquired by a Bio-Rad Radiance Plus confocal using the Lasersharp software package. Both wild-type and sifA mutant bacteria contain a plasmid (pFPV25.1) which provides constitutive expression of GFP (in green; yellow in overlap) under the rpsM promoter, as previously described (43). Rapid movement and replication of sifA mutant bacteria in the cytosol of infected cells were readily observed, in contrast to the immobile and nonreplicating bacteria in acidified vacuoles (arrows in left-hand series). Wild-type bacteria replicated in acidified vacuoles (arrows in right-hand series).
Based on these observations, we conclude that the previously observed enhanced replication of sifA bacteria in both HeLa and MDCK epithelial cells (39) occurs in the cytosol of these cell types. Thus, in contrast to the nonpermissive cytosolic environment of the macrophage (3), serovar Typhimurium can survive and replicate in the cytosol of epithelial cells. During the course of our studies, Goetz and colleagues demonstrated that serovar Typhimurium 14028s does not replicate in the epithelial cell line Caco-2 following direct delivery to the cytosol by microinjection (19). The discrepancy between their results and our own suggests that serovar Typhimurium requires expression of specific virulence factors which are induced following invasion and vacuolar adaptation (29) prior to release in the cytosol in order to replicate in this compartment.
In previous studies we have demonstrated that transfection of HeLa cells with an N-terminal fusion of SifA to GFP causes swelling and aggregation of endocytic compartments bearing lysosomal glycoproteins (5) and the late endosome marker lysobisphosphatidic acid (4). These observations suggest that SifA directs membrane fusion events for the purpose of maintaining vacuole integrity and for Sif formation. To examine a possible role for the host cell endocytic pathway in SCV maintenance, we treated cells with 100 nM wortmannin, a potent phosphatidylinositol 3-kinase inhibitor that impairs many endocytic and secretory processes (7, 8, 24, 27, 31, 35). Cells were then infected with wild-type serovar Typhimurium and examined by immunofluorescence microscopy. Treatment with wortmannin led to a significant increase in the number of cytosolic bacteria (as determined by loss of LAMP-1 [Fig. 3B ] and altered bacterial morphology) at 6 h postinvasion. Interestingly, Sif formation was not impaired by treatment with wortmannin (Fig. 3A), suggesting that the formation of Sif tubules (a process involving aggregation and fusion of late endocytic compartments [4]) and maintenance of the SCV are separable functions of SifA. The gentamicin resistance assay (38) was used to quantify intracellular replication under these conditions. Pretreatment for 30 min with wortmannin did not affect invasion (as previously demonstrated [25]) but consistently led to a two- to threefold increase in intracellular replication at 6 h postinvasion over that of untreated cells (37). Thus, pharmacologic inhibition of the host endosomal system can affect the intracellular location and replication efficiency of serovar Typhimurium in epithelial cell lines.
FIG. 3.
Effects of wortmannin on maintenance of the Salmonella-containing vacuole. (A) HeLa cells were infected for 6 h with wild-type serovar Typhimurium SL1344 in the presence of 100 nM wortmannin and then fixed with 2.5% paraformaldehyde. Cells were coimmunostained for LAMP-1 and LPS, as indicated, and processed for immunofluorescence microscopy. The arrow indicates LAMP-1− bacteria which are present in the cytosol and undergoing replication. Sif formation was not inhibited by wortmannin treatment; a representative Sif is indicated with an arrowhead. Size bar, 10 μm. (B) Quantitation of intracellular bacteria colocalizing with LAMP-1 for experiments performed as described for panel A. The averages ± standard errors are shown for three separate experiments. ∗, P < 0.001
We also examined the role of the host endosomal system in SCV maintenance by expression of Rab GTPases, key regulators of many endocytic trafficking events (36). To test a role for early endosomes in SCV maintenance, HeLa cells were transfected (5) with a vector encoding the Q79L (constitutively active) GTPase mutant of Rab5 fused to the C terminus of GFP (generously provided by C. Roy, Yale University). As previously demonstrated (40), expression of Rab5 Q79L caused extensive swelling of endosomes (Fig. 4A). Sifs were seen in only 11% ± 2% of Rab5 Q79L-transfected cells compared with 49% ± 5% of wild-type Rab5-GFP-transfected cells (n = 3). Expression of Rab5 Q79L also led to an increase in the number of transfected cells that contained cytosolic bacteria compared to the number of wild-type cells transfected with Rab5-GFP or GFP alone, indicating a loss of SCV integrity (Fig. 4B). This is consistent with a recent report demonstrating that expression of Rab5Q79L leads to an increase in intracellular replication by serovar Typhimurium in HeLa epithelial cells at 5.5 h postinvasion (2). The authors of that study concluded that intracellular replication can be uncoupled from normal maturation of the SCV. In light of our findings, we propose instead that the increase in replication observed by Baldeón et al. occurs in the cytosol of infected cells as a result of altering Rab5 activity. However, our results and those of Baldeón et al. (2) demonstrate a role for membrane traffic involving early endosomes for maintenance of the SCV and for Sif formation.
FIG. 4.
Expression of Rab5 Q79L blocks Sif formation and promotes the release of serovar Typhimurium from the Salmonella-containing vacuole. (A) HeLa cells were transfected with a vector encoding the Q79L mutant of Rab5 fused to GFP prior to infection for 6 h with wild-type serovar Typhimurium SL1344. Cells were then fixed with 2.5% paraformaldehyde, coimmunostained for LAMP-1 and LPS, and processed for immunofluorescence microscopy. Arrowheads indicated swollen endocytic structures that contain LAMP-1, while the arrow indicates cytosolic bacteria lacking LAMP-1 that are undergoing replication. Size bar, 10 μm. (B) HeLa cells were transfected with a vector encoding either the enhanced GFP alone or its fusion to the indicated Rab GTPase. Transfected cells were infected for 6 h with wild-type serovar Typhimurium and then fixed with 2.5% paraformaldehyde. Fixed cells were coimmunostained for LAMP-1 and LPS, and the number of transfected cells with large clusters of LAMP-1− bacteria, as demonstrated for panel A, was determined. The averages ± standard errors are shown for three separate experiments. ∗, P < 0.001.
To test a role for late endocytic compartments in maintenance of the SCV, HeLa cells were transfected with a vector encoding the N125I (dominant-negative) mutant of Rab7 fused to the C terminus of GFP (generously provided by A. Wandinger-Ness, University of New Mexico Health Sciences Center). Expression of this construct inhibits Sif formation (4) and also led to an increase in the number of transfected cells with cytosolic bacteria (Fig. 4B). Thus, interruption of both early (Rab5-mediated) and late (Rab7-mediated) stages of the endocytic pathway lead to interruption of the normal membrane traffic events that allow Sif formation and preservation of the SCV.
Many studies utilize epithelial cell lines to examine intracellular replication by serovar Typhimurium. Here we demonstrate that genetic, pharmacologic, or molecular interference with this model of infection can lead to an alteration in the subcellular localization of intracellular bacteria. Since we have shown that serovar Typhimurium can replicate efficiently in the cytosol of epithelial cells, suitable care must be taken to ensure that vacuolar replication is not confused with cytosolic replication. It is interesting that serovar Typhimurium actively maintains the SCV (through the actions of SifA) in epithelial cells when clearly it could benefit by escaping this compartment. Indeed, vacuolar escape and cytosolic replication constitute the pathogenic strategy of such pathogens as Listeria monocytogenes and Shigella flexneri. Maintenance of the SCV in epithelial cells may have important implications for host immune responses, including TAP (transporter associated with antigen presentation)-dependent presentation of antigens via major histocompatibility complex class I molecules and activation of proinflammatory signaling cascades in response to intracellular LPS (18). In macrophages, maintenance of the SCV also allows serovar Typhimurium to avoid cytosolic host defense proteins such as ubiquicidin, which has the ability to restrict bacterial growth in vitro (22). Future studies will address how serovar Typhimurium modifies its vacuolar compartment to allow replication within this intracellular niche.
Acknowledgments
We thank members of the Finlay lab and Olivia Steele-Mortimer for careful reading of the manuscript. We also thank Elaine Humphrey of the Electron Microscopy Lab, University of British Columbia, for her assistance with confocal microscopy. Special thanks to C. Roy and A. Wandinger-Ness for providing necessary reagents and helpful advice and to R. Valdivia, S. Falkow, and D. Holden for providing plasmid pFPV25.1.
This work was supported by grants (to B.B.F.), a postdoctoral fellowship (to J.H.B.), and a doctoral research award (to M.L.Z.) from the Canadian Institute of Health Research. B.B.F. is an International Research Scholar of the Howard Hughes Medical Institute and a Distinguished Investigator of the Canadian Institute for Health Research. J.H.B. is an honorary fellow of the Izaac Walton Killam Memorial Foundation. M.L.Z. is the recipient of a War Memorial Scholarship from the National Chapter of Canada Imperial Order of the Daughters of the Empire.
Editor: A. D. O'Brien
REFERENCES
- 1.Alpuche Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 89:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldeon, M. E., B. P. Ceresa, and J. E. Casanova. 2001. Expression of constitutively active Rab5 uncouples maturation of the Salmonella-containing vacuole from intracellular replication. Cell. Microbiol. 3:473-486. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumell, J., P. Tang, S. Mills, and B. Finlay. 2001. Characterization of Salmonella-induced filaments (Sifs) reveals a delayed interaction between Salmonella-containing vacuoles and late endocytic compartments. Traffic 2:643-653. [DOI] [PubMed] [Google Scholar]
- 5.Brumell, J. H., C. M. Rosenberger, G. T. Gotto, S. L. Marcus, and B. B. Finlay. 2001. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell. Microbiol. 3:75-84. [DOI] [PubMed] [Google Scholar]
- 6.Brumell, J. H., O. Steele-Mortimer, and B. B. Finlay. 1999. Bacterial invasion: force feeding by Salmonella. Curr. Biol. 9:R277-R280. [DOI] [PubMed] [Google Scholar]
- 7.Clague, M. J., C. Thorpe, and A. T. Jones. 1995. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 367:272-274. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, H. W. 1995. Wortmannin causes mistargeting of procathepsin D: evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol. 130:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell. Microbiol. 2:365-377. [DOI] [PubMed] [Google Scholar]
- 10.Finlay, B. B., B. Gumbiner, and S. Falkow. 1988. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J. Cell Biol. 107:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galan, J. E., and D. Zhou. 2000. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl. Acad. Sci. USA 97:8754-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-del Portillo, F., and B. B. Finlay. 1995. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J. Cell Biol. 129:81-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-del Portillo, F., J. W. Foster, M. E. Maguire, and B. B. Finlay. 1992. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol. Microbiol. 6:3289-3297. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-del Portillo, F., M. A. Stein, and B. B. Finlay. 1997. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect. Immun. 65:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-del Portillo, F., M. B. Zwick, K. Y. Leung, and B. B. Finlay. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA 90:10544-10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia Vescovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 18.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz, M., A. Bubert, G. Wang, I. Chico-Calero, J. A. Vazquez-Boland, M. Beck, J. Slaghuis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groisman, E. A. 1998. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays 20:96-101. [DOI] [PubMed] [Google Scholar]
- 21.Guy, R. L., L. A. Gonias, and M. A. Stein. 2000. Aggregation of host endosomes by Salmonella requires SPI2 translocation of SseFG and involves SpvR and the fms-aroE intragenic region. Mol. Microbiol. 37:1417-1435. [DOI] [PubMed] [Google Scholar]
- 22.Hiemstra, P. S., M. T. van den Barselaar, M. Roest, P. H. Nibbering, and R. van Furth. 1999. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 66:423-428. [DOI] [PubMed] [Google Scholar]
- 23.Jones, B. D., and S. Falcow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 24.Li, G., C. D'Souza-Schorey, M. A. Barbieri, R. L. Roberts, A. Klippel, L. T. Williams, and P. D. Stahl. 1995. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA 92:10207-10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mecsas, J., B. Raupach, and S. Falkow. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269-1281. [DOI] [PubMed] [Google Scholar]
- 26.Meresse, S., O. Steele-Mortimer, B. B. Finlay, and J. P. Gorvel. 1999. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninomiya, N., K. Hazeki, Y. Fukui, T. Seya, T. Okada, O. Hazeki, and M. Ui. 1994. Involvement of phosphatidylinositol 3-kinase in Fc gamma receptor signaling. J. Biol. Chem. 269:22732-22737. [PubMed] [Google Scholar]
- 28.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeifer, C. G., S. L. Marcus, O. Steele-Mortimer, L. A. Knodler, and B. B. Finlay. 1999. Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect. Immun. 67:5690-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reaves, B. J., N. A. Bright, B. M. Mullock, and J. P. Luzio. 1996. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J. Cell Sci. 109:749-762. [DOI] [PubMed] [Google Scholar]
- 32.Richter-Dahlfors, A., A. M. J. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 3:587-598. [DOI] [PubMed] [Google Scholar]
- 34.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd, P. R., M. A. Soos, and K. Siddle. 1995. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 211:535-539. [DOI] [PubMed] [Google Scholar]
- 36.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113:183-192. [DOI] [PubMed] [Google Scholar]
- 37.Steele-Mortimer, O., J. H. Brumell, L. A. Knodler, S. Meresse, A. Lopez, and B. B. Finlay. 2002. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell. Microbiol. 4:43-54. [DOI] [PubMed] [Google Scholar]
- 38.Steele-Mortimer, O., S. Méresse, J.-P. Gorvel, B.-H. Toh, and B. B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell. Microbiol. 1:33-51. [DOI] [PubMed] [Google Scholar]
- 39.Stein, M. A., K. Y. Leung, M. Zwick, F. Garcia-del Portillo, and B. B. Finlay. 1996. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol. Microbiol. 20:151-164. [DOI] [PubMed] [Google Scholar]
- 40.Stenmark, H., R. G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsolis, R. M., R. A. Kingsley, S. M. Townsend, T. A. Ficht, L. G. Adams, and A. J. Baumler. 1999. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473:261-274. [PubMed] [Google Scholar]
- 42.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez-Torres, A., G. Fantuzzi, C. K. Edwards III, C. A. Dinarello, and F. C. Fang. 2001. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc. Natl. Acad. Sci. USA 98:2561-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]