Abstract
Arginine-specific cysteine proteinases (gingipains-R) from periodontopathic Porphyromonas gingivalis cleaved CD14, a bacterial pattern recognition receptor, on human gingival fibroblasts (HGF). Consequently, gingipains-R reduced lipopolysaccharide-induced interleukin-8 production by HGF, indicating that gingipains-R inhibited CD14-dependent HGF activation and are involved in immune evasion by the bacterium in periodontal tissues.
Porphyromonas gingivalis has been implicated as a principal bacterium in adult periodontitis (8, 30) and possesses a number of putative virulence factors such as proteolytic enzymes (3). It produces two cysteine proteinases specific for Arg-X (50 and 95 kDa) or Lys-X (105 kDa) bonds, which are referred to as arginine-specific gingipain (Rgp) and lysine-specific gingipain (Kgp), respectively (2, 23). The 95-kDa high-molecular-mass Rgp (HRgpA) differs from the 50-kDa Rgp (RgpB) in that the protein noncovalently complexes with the hemagglutinin/adhesin domain in the same manner as Kgp. It has been shown that gingipains play a critical role in the onset of inflammation through a wide variety of biological activities (9-17, 19, 20, 32, 38).
CD14, a 55-kDa glycosylphosphatidylinositol (GPI)-anchored membrane protein, functions as a pattern recognition receptor for many bacterial components such as lipopolysaccharide (LPS) in the innate immune response (27, 39); e.g., CD14 mediates sensitive responses to LPS by interacting with Toll-like receptor 4 (TLR4) and MD-2, a molecule associated with TLR4 (25, 28, 31, 35). CD14 is expressed strongly on monocytes (5, 36) and weakly on neutrophils (7) and human gingival fibroblasts (HGF) (33, 37), all of which exist in periodontal tissue with periodontitis, indicating that these cell types are the first line of defense against invasive bacteria triggered by the bacterial components via CD14 in periodontal tissues.
We have recently shown that the gingipains preferentially cleave CD14 on human monocytes and consequently inhibit a CD14-dependent monocyte activation pathway triggered by LPS (32), suggesting that P. gingivalis could evade immune surveillance controlled by monocytes. Periodontitis is clinically characterized as inflammation in periodontal connective tissue, in which the dominant cell type is HGF. HGF may actively participate in the inflammatory response by producing various cytokines (34) and chemokines such as interleukin-8 (IL-8) (33) which are released from HGF via CD14. Therefore, we examined the effect of gingipains on HGF functions.
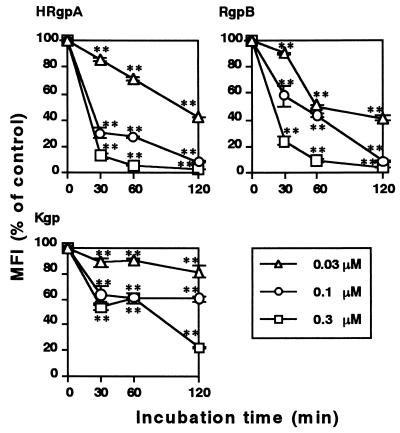
We first examined the effect of gingipains purified from P. gingivalis HG66 culture supernatant (23, 26) on the expression of CD14 by HGF by using flow cytometry (32). HGF were prepared from the explants of normal gingiva obtained from patients who gave informed consent (33). When HGF were treated with 0.03 to 0.3 μM HRgpA and RgpB for 30 min, the expression of CD14 on the cell surface was significantly (P < 0.01) reduced (Fig. 1). The expression was almost completely abolished by 60 to 120 min of treatment with 0.1 and 0.3 μM concentrations of both HRgpA and RgpB. By contrast, Kgp exhibited much less activity for the reduction than Rgps. An Rgp-specific inhibitor, Phe-Pro-Arg-chloromethyl ketone (FPR-cmk) (32), efficiently inhibited HRgpA activity at a 10-fold molar excess (Table 1). By contrast, a Kgp-specific inhibitor, benzyloxycarbonyl-Phe-Lys-chloromethyl ketone (32), exerted only a marginal effect, indicating that the enzymatic activity of Rgp is required for the elimination of CD14. HRgpA differs from RgpB in that it has a hemagglutinin/adhesin domain. This domain binds to fibrinogen, fibronectin, and laminin (24) and reacts with phospholipids of the plasma membrane in a Ca2+-dependent manner (15). This could be the reason that HRgpA cleaved CD14 more efficiently than RgpB, i.e., because the domain might increase the affinity of the enzyme ligand. HRgpA at 1.0 μM still effectively reduced CD14 expression in the presence of 20% freshly isolated human serum (Table 1), indicating that high doses of gingipains are resistant to inhibitors in serum. This observation is supported by the fact that serum is ineffective in preventing the Rgp activation of prekallikrein (11), factor X (14), and protein C (9).
FIG. 1.
Kinetics of CD14 reduction on HGF caused by gingipain treatment. HGF were treated with the indicated concentrations of HRgpA, RgpB, and Kgp for the indicated times at 37°C. After treatment, cells were washed with phosphate-buffered saline and stained with anti-human CD14 MEM-18 or isotype-matched MAb and analyzed by flow cytometry. Representative findings of three independent experiments are expressed as the means of the mean fluorescence intensities (MFI) (% of control) (32). ∗∗, P < 0.01 versus respective untreated cells at each time point by a one-way analysis of variance.
TABLE 1.
Effect of gingipain-specific inhibitors and serum on the reduction of CD14 on HGF induced by HRgpAa
| HRgpA concn (μM) | Inhibitor | Inhibitor concn | Binding of MEM-18 (%)b | % Inhibitionb |
|---|---|---|---|---|
| 0.3 | 25.7 ± 0.2 | |||
| 0.3 | FPR-cmk | 3 μM | 97.6 ± 0.4 | 96.7∗∗ |
| 0.3 | Z-FK-cmkc | 3 μM | 43.7 ± 4.6 | 24.5∗∗ |
| 1.0 | 0.4 ± 0.4 | |||
| 1.0 | Human serum | 1% | 0.9 ± 0.6 | 0.5 |
| 1.0 | Human serum | 10% | 16.9 ± 0.2 | 16.6∗∗ |
| 1.0 | Human serum | 20% | 28.4 ± 0.4 | 28.1∗∗ |
HRgpA at a dose indicated was pretreated with or without gingipain-specific inhibitors or freshly isolated human serum at the indicated concentrations for 10 min at room temperature. HGF were then treated with the supernatants for 30 min at 37°C.
After the treatment, cells were stained with MEM-18 or isotype-matched MAb and analyzed by flow cytometry. Representative findings of three independent experiments are expressed as the means of the MFI and expressed as percent binding and percent inhibition. ∗∗, P < 0.01 for combined HRgpA and RgpB treatment versus HRgpA treatment alone at 0.3 and 1.0 μM, respectively.
Z-FK-cmk, benzyloxycarbonyl-Phe-Lys-chloromethyl ketone.
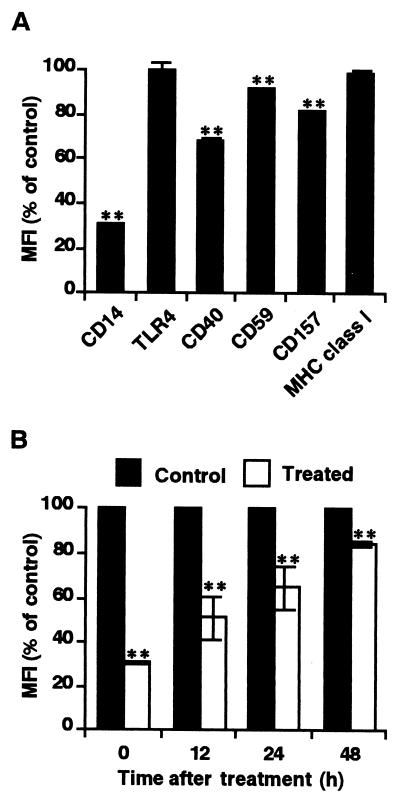
Expression of CD59 and CD157, two other GPI-anchored proteins (4, 18), as well as CD40 was only slightly decreased by treatment with HRgpA (Fig. 2A), which eliminated the possibility that Rgp preferentially cleaves GPI-anchored molecules on the cell surface. The expression of TLR4 as well as of major histocompatibility complex class I molecules was unchanged after the treatment, although there are 21 Arg and 9 Lys sites and 29 Arg and 35 Lys sites on CD14 and TLR4 amino acid sequences, respectively (6, 29). These findings indicate that HRgpA is structurally inaccessible to TLR4 and that at least the functional anti-TLR4 binding site is conserved. After treatment, CD14 was gradually reexpressed on the cell surface upon reculture and more than 80% of the initial value was recovered at 48 h (Fig. 2B).
FIG. 2.
Preferential reduction of CD14 on HGF by HRgpA and reexpression of CD14 after HRgpA treatment. HGF were treated with 0.3 μM HRgpA for 30 min. (A) CD14, TLR4, CD40, CD59, CD157, and major histocompatibility complex class I (MHC class I) expressions on HGF were measured by flow cytometry. Representative findings of three independent experiments are expressed as the means of the MFI (% of control). ∗∗, P < 0.01 versus respective untreated cells. (B) After being washed with medium three times, cells were recultured in alpha-minimum essential medium supplemented with 10% fetal calf serum for the periods indicated. After being harvested by trypsinization, cells were stained with MEM-18 and analyzed by flow cytometry. The findings are expressed as percentages of the MFI of the control cells indicated versus those for the same time points without pretreatment with HRgpA. Representative findings of three independent experiments are expressed as the means of the MFI (% of control). ∗∗, P < 0.01 versus respective untreated cells at each time point. Error bars, standard deviations.
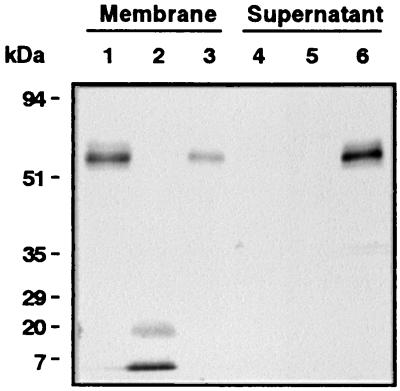
Immunoblot analysis using anti-human CD14 polyclonal antibody (32) showed that pretreatment of the purified membrane fraction isolated from HGF (33) with HRgpA markedly degraded CD14 into multiple CD14 fragments (Fig. 3), indicating that the reduction of CD14 on HGF induced by gingipains resulted from direct proteolysis and that CD14 proteolyzed by gingipains had no ability to function as the soluble form. Gingipains can also degrade soluble CD14 (32). Therefore, it is conceivable that gingipains down-regulate CD14-mediated cell activation in vivo.
FIG. 3.
Immunoblot analysis of CD14 expressed on and released by HGF after treatment with HRgpA. Purified cell membrane of HGF was untreated (lanes 1 and 4) or treated with 0.3 μM HRgpA (lanes 2 and 5) or 5-U/ml phosphatidylinositol-specific phospholipase C as a positive control (lanes 3 and 6) for 1 h at 37°C. After the cell membrane and the supernatant were separated by ultracentrifugation, both specimens were solubilized with Laemmli sample buffer (10% glycerol, 1% sodium dodecyl sulfate, 0.0025% bromphenol blue, 50 mM Tris-HCl [pH 6.8]), subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. The blot was probed with an anti-CD14 polyclonal antibody. Molecular mass markers are shown on the left. Findings are representative of four independent experiments.
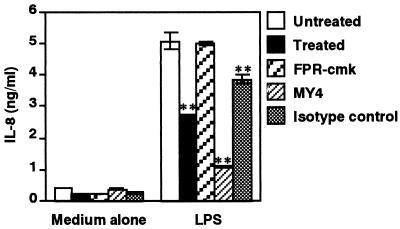
In support of this hypothesis, preincubation of HGF with 0.3 μM HRgpA for 30 min markedly suppressed IL-8 production triggered by 10 ng of LPS/ml (Escherichia coli O55:B5; Sigma), as determined by enzyme-linked immunosorbent assay (22) (Fig. 4). Pretreatment of 0.3 μM HRgpA with 3 μM FPR-cmk for 10 min recovered IL-8 production. Anti-CD14 monoclonal antibody (MAb) was used as a positive control. Furthermore, preincubation of HGF with 0.3 μM HRgpA for 30 min did not have any inhibitory effect on IL-8 production by HGF upon stimulation with phorbol myristate acetate (data not shown). In the present study, stimulation of HRgpA-treated HGF with LPS after removal of residual HRgpA by washing (Fig. 4) eliminated the possibility that the decrease in IL-8 production was due to degradation of IL-8 by HRgpA (21). IL-8 is one of the major chemokines produced by HGF triggered by LPS via CD14 (33). IL-8 triggers both neutrophil degranulation and respiratory burst and enhances phagocytosis in addition to chemotactic activity (1, 25), indicating that reduced production of IL-8 may be important for attenuating neutrophil function. Collectively, the data reported here indicate that Rgps downregulates CD14-mediated cell activation of HGF and further support the hypothesis of the immune evasion mechanism of P. gingivalis in periodontal tissues.
FIG. 4.
Inhibition of LPS-induced IL-8 production in HGF by HRgpA treatment. HGF monolayer cells were treated with 0.3 μM HRgpA for 30 min at 37°C or with anti-CD14 MY4 (10 μg/ml) or isotype-matched control immunoglobulin G2b (10 μg/ml) before stimulation with 10 ng of LPS/ml for 4 h at 37°C in alpha-minimum essential medium supplemented with 1% fetal calf serum. The amount of IL-8 in the culture supernatants was analyzed by enzyme-linked immunosorbent assay. Representative findings of three independent experiments are shown as the means ± standard deviations of triplicate assays. ∗∗, P < 0.01 versus respective control.
Acknowledgments
We thank K. Miyake and S. Akashi (The University of Tokyo, Tokyo, Japan) for providing anti-TLR4 MAb HTA1216.
This work was supported in part by Grants-in-Aid for Scientific Research (12470380 and 13671894) and for Encouragement of Young Scientists (12771320) from the Japan Society for the Promotion of Science.
Editor: J. D. Clements
REFERENCES
- 1.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 2.Chen, Z., J. Potempa, A. Polanowski, M. Wikstrom, and J. Travis. 1992. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J. Biol. Chem. 267:18896-18901. [PubMed] [Google Scholar]
- 3.Cutler, C. W., J. R. Kalmar, and C. A. Genco. 1995. Pathogenic strategies of oral anaerobe. Porphyromonas gingivalis. Trends Microbiol. 3:45-51. [DOI] [PubMed] [Google Scholar]
- 4.Davies, A., D. L. Simmons, G. Hale, R. A. Harrison, H. Tighe, P. J. Lachmann, and H. Waldmann. 1989. CD59, an LY6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J. Exp. Med. 170:637-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin, J. D., J. Ritz, L. M. Nadler, and S. F. Schlossman. 1981. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J. Clin. Investig. 68:932-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haziot, A., S. Chen, E. Ferrero, M. G. Low, R. Silber, and S. M. Goyert. 1988. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 141:547-552. [PubMed] [Google Scholar]
- 7.Haziot, A., B.-Z. Tsuberi, and S. M. Goyert. 1993. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-α in response to lipopolysaccharide. J. Immunol. 150:5556-5565. [PubMed] [Google Scholar]
- 8.Holt, S. C., and T. E. Bramanti. 1991. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit. Rev. Oral Biol. Med. 2:177-281. [DOI] [PubMed] [Google Scholar]
- 9.Hosotaki, K., T. Imamura, J. Potempa, N. Kitamura, and J. Travis. 1999. Activation of protein C by arginine-specific cysteine proteinases (gingipains-R) from Porphyromonas gingivalis. Biol. Chem. 380:75-80. [DOI] [PubMed] [Google Scholar]
- 10.Imamura, T., A. Banbula, P. J. B. Pereira, J. Travis, and J. Potempa. 2001. Activation of human prothrombin by arginine-specific cysteine proteinases (gingipains R) from Porphyromonas gingivalis. J. Biol. Chem. 276:18984-18991. [DOI] [PubMed] [Google Scholar]
- 11.Imamura, T., R. N. Pike, J. Potempa, and J. Travis. 1994. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J. Clin. Investig. 94:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura, T., J. Potempa, R. N. Pike, J. N. Moore, M. H. Barton, and J. Travis. 1995. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infect. Immun. 63:4877-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamura, T., J. Potempa, R. N. Pike, and J. Travis. 1995. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect. Immun. 63:1999-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura, T., J. Potempa, S. Tanase, and J. Travis. 1997. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J. Biol. Chem. 272:16062-16067. [DOI] [PubMed] [Google Scholar]
- 15.Imamura, T., J. Potempa, and J. Travis. 2000. Comparison of pathogenic properties between two types of arginine-specific cysteine proteinases (gingipains-R) from Porphyromonas gingivalis. Microb. Pathog. 29:155-163. [DOI] [PubMed] [Google Scholar]
- 16.Imamura, T., S. Tanase, T. Hamamoto, J. Potempa, and J. Travis. 2001. Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis. Biochem. J. 353:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagels, M. A., J. Travis, J. Potempa, R. Pike, and T. E. Hugli. 1996. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect. Immun. 64:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaisho, T., J. Ishikawa, K. Oritani, J. Inazawa, H. Tomizawa, O. Muraoka, T. Ochi, and T. Hirano. 1994. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc. Natl. Acad. Sci. USA 91:5325-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lourbakos, A., J. Potempa, J. Travis, M. R. D'Andrea, P. Andrade-Gordon, R. Santulli, E. J. Mackie, and R. N. Pike. 2001. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect. Immun. 69:5121-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lourbakos, A., Y. Yuan, A. L. Jenkins, J. Travis, P. Andrade-Gordon, R. Santulli, J. Potempa, and R. N. Pike. 2001. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood 97:3790-3797. [DOI] [PubMed] [Google Scholar]
- 21.Mikolajczyk-Pawlinska, J., J. Travis, and J. Potempa. 1998. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 440:282-286. [DOI] [PubMed] [Google Scholar]
- 22.Nemoto, E., S. Sugawara, H. Tada, H. Takada, H. Shimauchi, and H. Horiuchi. 2000. Cleavage of CD14 on human gingival fibroblasts cocultured with activated neutrophils is mediated by human leukocyte elastase resulting in down-regulation of lipopolysaccharide-induced IL-8 production. J. Immunol. 165:5807-5813. [DOI] [PubMed] [Google Scholar]
- 23.Pike, R., W. McGraw, J. Potempa, and J. Travis. 1994. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis: isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 269:406-411. [PubMed] [Google Scholar]
- 24.Pike, R. N., J. Potempa, W. McGraw, T. H. Coetzer, and J. Travis. 1996. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J. Bacteriol. 178:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 26.Potempa, J., R. Pike, and J. Travis. 1997. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 378:223-230. [DOI] [PubMed] [Google Scholar]
- 27.Pugin, J., D. Heumann, A. Tomasz, V. V. Kravchenko, Y. Akamatsu, M. Nishijima, M. P. Glauser, P. S. Tobias, and R. J. Ulevitch. 1994. CD14 is a pattern recognition receptor. Immunity 1:509-516. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi, S. T., L. Larivière, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah, H. N., D. Mayrand, and R. Genco (ed.) 1993. Biology of the species Porphyromonas gingivalis. CRC Press, Boca Raton, Fla.
- 31.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugawara, S., E. Nemoto, H. Tada, K. Miyake, T. Imamura, and H. Takada. 2000. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J. Immunol. 165:411-418. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara, S., A. Sugiyama, E. Nemoto, H. Rikiishi, and H. Takada. 1998. Heterogeneous expression and release of CD14 by human gingival fibroblasts: characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect. Immun. 66:3034-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada, H., J. Mihara, I. Morisaki, and S. Hamada. 1991. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect. Immun. 59:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 36.Todd, R. F., L. M. Nadler, and S. F. Schlossman. 1981. Antigens on human monocytes identified by monoclonal antibodies. J. Immunol. 126:1435-1442. [PubMed] [Google Scholar]
- 37.Watanabe, A., A. Takeshita, S. Kitano, and S. Hanazawa. 1996. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect. Immun. 64:4488-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingrove, J. A., R. G. DiScipio, Z. Chen, J. Potempa, J. Travis, and T. E. Hugli. 1992. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J. Biol. Chem. 267:18902-18907. [PubMed] [Google Scholar]
- 39.Wright, S. D. 1995. CD14 and innate recognition of bacteria. J. Immunol. 155:6-8. [PubMed] [Google Scholar]