Abstract
Brucella melitensis is a gram-negative alpha2-proteobacterium responsible for abortion in goats and for Malta fever in humans. This facultative intracellular pathogen invades and survives within both professional and nonprofessional phagocytes. A dichloromethane extract of spent culture supernatant from B. melitensis induces bioluminescence in an Escherichia coli acyl-homoserine lactone (acyl-HSL) biosensor strain based upon the activity of the LasR protein of Pseudomonas aeruginosa. HPLC fractionation of the extract, followed by mass spectrometry, identified the major active molecule as N-dodecanoylhomoserine lactone (C12-HSL). This is the first report of the production of an acyl-HSL by an intracellular pathogen. The addition of synthetic C12-HSL to an early log phase culture of either B. melitensis or Brucella suis 1330 reduces the transcription of the virB operon, which contains virulence genes known to be required for intracellular survival. This mimics events seen during the stationary phase of growth and suggests that quorum sensing may play a role in the control of virulence in Brucella.
Brucella is the causative agent of brucellosis, a widely distributed zoonosis affecting a broad range of mammals and causing a chronic undulant fever in humans (reviewed by Smith and Ficht [53]). Brucellosis remains a health problem and a source of major economic losses in developing countries, where it is endemic. Brucella are nonmotile gram-negative bacteria and facultative intracellular pathogens that are able to invade and replicate in macrophages and nonprofessional phagocytes (12). In HeLa cells, virulent Brucella cells alter the intracellular trafficking of membrane-bound intracellular compartments to create a novel vacuolar niche in which they replicate (41). The mechanisms and the factors involved in this process are not well understood, with knowledge about the interactions between Brucella and the mammalian cell being mainly descriptive.
Molecular genetic analyses have identified several types of factors that are important for Brucella-host cell interactions: structural components like lipopolysaccharide (1, 16, 32) and cyclic β(1-2) glucan (19), stress response proteins like DnaK (23) and Hfq, a homologue of Escherichia coli host factor I (HF-I) (43), and also a type IV secretion system (14, 34, 51). The VirB type IV secretion system is homologous to the VirB system of Agrobacterium tumefaciens and to the Ptl system of Bordetella pertussis (4). DNA sequence analysis of the Brucella suis virB operon has revealed 12 open reading frames (ORFs) encoding homologues of the 11 VirB proteins encoded by the pTi plasmid of Agrobacterium and a 12th ORF encoding a putative lipoprotein (34). Independent mutants of virB5, virB9, or virB10 are highly attenuated in an in vitro infection model of human macrophages (34). Two recent signature-tagged mutagenesis studies have confirmed the requirement of virB2, virB4, and virB8 for survival within macrophages and HeLa cells (14) and of virB1 and virB10 in the early stages of chronic infection in BALB/c mice (18).
The process of infection requires a complex and multifactorial regulation of virulence factors. One of the most abundant types of virulence regulatory system is the two-component family of signal transducers (7). Two-component regulatory systems that have recently been described are involved in the virulence of Brucella: the BvrR/BvrS system is critical for the intracellular growth of Brucella abortus (54), and a second system, homologous to VsrB/VsrC of Ralstonia solanacearum, has been identified in Brucella melitensis in a signature-tagged mutagenesis screening in the mouse infection model (27). If two-component regulation systems allow each single bacterium within the population to react in face of environmental changes, other systems, such as the quorum sensing, respond to signals produced by the bacteria themselves and control virulence factors at the whole-population level.
Quorum sensing (QS) is a regulatory system for controlling gene expression in response to increasing cell density (reviewed in references 13, 15, 58, and 66). This cell-to-cell signaling is achieved through the production and release of a small signaling molecule or pheromone. With the growth of the bacterial population, there is an accumulation of this pheromone until a threshold concentration is reached, indicating that a quorum of bacteria is present. At this point, the pheromone activates a transcriptional regulator, leading to the activation or, in some cases, the repression of target genes (49, 55, 64, 65, 70). In a large number of gram-negative bacterial species, the signal molecule is an N-acyl homoserine lactone (acyl-HSL). In most cases, the acyl-HSL synthase is a member of the LuxI family and the response regulator is a member of the LuxR family (reviewed by Swift [59]). Here, the concentration of the signal molecule reflects the numbers of bacterial cells and the perception of a threshold level of the signal indicates a quorate population, ready to employ multicellular adaptive responses involved in, for example, virulence (21, 37, 46, 64), symbiosis (29, 63), and biofilm formation (10, 30) as well as individual survival strategies, such as the induction of stationary phase responses (25) and motility for colony escape (2, 42).
Acyl-HSL signals are composed of a homoserine lactone ring linked via an amide bond to an acyl chain of variable length and with variable substitutions. In vitro and in vivo studies have demonstrated that acyl-HSL synthesis couples amino acid and fatty acid biosynthesis, with the HSL moiety derived from S-adenosyl methionine and the acyl moiety from acylated-acyl carrier protein (acyl-ACP) (20, 33, 35, 48, 61). Signals with shorter acyl chains (C4 and C6) are freely diffusible (22, 38); however, in Pseudomonas aeruginosa, the long acyl chain signal (3-oxo-C12-HSL) requires an active efflux pump for its release, although it may enter cells by diffusion (38).
Surprisingly, acyl-HSL signals with long acyl chains exert significant effects upon the cells and tissues of eukaryotic organisms. An immunosuppressive effect of 3-oxo-C12-HSL upon the host immune system has been observed by Telford et al. (60), an effect that will prejudice the host against antibacterial action and so favor bacteria. Additionally, Lawrence et al. have described a vasorelaxant activity for 3-oxo-C12-HSL, which may contribute to the ability of the bacteria to maintain the supply of key nutrients to a site of infection by increasing the local blood flow (26).
The advantages to an intracellular pathogen of using QS to regulate genes within the closed environment of the replication vacuole and also to be able to modulate the activity of the host's immune responses are clear. We therefore sought to determine whether Brucella employs acyl-HSL-mediated QS, and in this paper, we report the identification and purification of N-dodecanoyl-homoserine lactone (C12-HSL) and its role in the control of the transcription of the virB operon, thus suggesting a putative link between QS and virulence in Brucella.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Table 1 describes the bacterial strains and plasmids used in this study. E. coli and Chromobacterium violaceum strains were routinely cultured at 30°C in Luria-Bertani (LB) medium (NaCl, 10 g liter−1; yeast extract, 5 g liter−1; peptone, 10 g liter−1) supplemented with tetracycline (20 μg ml−1) where appropriate. Brucella strains were routinely cultured in 2YT medium (NaCl, 5 g liter−1; yeast extract, 10 g liter−1; peptone, 10 g liter−1). For acyl-HSL extraction, a liquid culture of Brucella sp. was grown at 37°C in RPMI 1640 medium (Life Technologies, Merelbeke, Belgium) supplemented with 0.1% glucose (RPMI-glucose). 2YT medium, yeast extract solution, and peptone solution used in competition assays with HSL were not autoclaved but were sterile filtered. As high temperature may promote the formation of cyclic dipeptides (diketopiperazines) (17), this ensured that inhibitory compounds found in these solutions were not the result of such treatment. To examine the influence of acyl-HSLs upon virB expression, B. melitensis 16M Nalr and B. suis were grown in modified minimal E medium (24).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| B. melitensis 16M Nalr | Spontaneous nalidixic acid-resistant mutant of the reference strain B. melitensis 16 M (Brucella Culture Collection of Nouzilly) | 62 |
| B. suis 1330 | B. suis biovar 1; reference strain (Brucella Culture Collection of Nouzilly) | ATCC 23444 |
| C. violaceum CV026 | Mini-Tn5 mutant derived from C. violaceum ATCC 31532 Hgr, cvil::Tn5xylE, Kmr, plus spontaneous Smr, acyl-HSL biosensor producing a purple pigment | 31 |
| E. coli JM109 | F′ traD36, proAB, laclq, lacZΔM15/recA1, endA1, gyrA96, thi, hsdR17, supE44, relA1, Δ(lac-proAB), mcrA | Promega |
| E. coli S17/1 λpir | λ-pir lysogen of S17-1 (thi pro hsdR−hsdM+recA RP4 2-Tc::Mu-Km::Tn7(Tpr Smr) | 52, 57 |
| Plasmids | ||
| pSB401 | luxR luxl′ (Photobacterium fischeri [ATCC 7744])::luxCDABE (Photorhabdus luminescens [ATCC 29999]) fusion; pACYC184-derived, Tcr, acyl-HSL biosensor producing bioluminescence | 68 |
| pSB1075 | lasR lasl′ (P. aeruginosa PAO1)::luxCDABE (P. luminescens [ATCC 29999]) fusion in pUC18 Apr, acyl-HSL biosensor producing bioluminescence | 68 |
Extraction, analysis, and detection of acyl-HSLs.
B. melitensis 16M Nalr was grown to stationary phase (96 h). Bacteria were removed from the culture by centrifugation at 3,000 × g for 10 min followed by filtration (0.2-μm pore size). The spent, cell-free supernatant from stationary phase cultures of B. melitensis 16M Nalr grown in RPMI glucose was extracted three times with dichloromethane (supernatant-to-dichloromethane ratio of 700:300). The dried extract was reconstituted in acetonitrile, and samples were subjected to analytical thin-layer chromatography (TLC) and preparative high-performance liquid chromatography (HPLC). TLC analysis was carried out on normal phase Silica gel 60F254 (Merck) using a solvent system of 45% hexane in acetone. Once resolved, the plate was dried and overlaid with a thin film of the biosensor E. coli (pSB1075) in 0.3% LB agar (31, 50, 68). After incubation at 30°C, acyl-HSLs were visualized either by autoradiography or photon camera imaging (Berthold LB980 photon video camera; EG&G Berthold U.K. Ltd., Milton Keynes, United Kingdom). A tentative identification of an acyl-HSL can be made by comparing the Rf values of the unknown with that of synthetic acyl-HSL standards. For preparative HPLC, samples were separated using a Kromasil KR100-5C8 (250 by 8 mm) reverse-phase column (Hichrom, Reading, United Kingdom) with a linear gradient of acetonitrile in water (20 to 100%) at a flow rate of 2 ml min−1 over a 37-min period. Seven fractions (F1 to F7) were collected, based on the retention time of the known acyl-HSLs and assayed using the acyl-HSL biosensor strains. Positive fractions were identified in agar plate assays and microplate assays as detailed by McClean et al. and Winson et al., respectively (31, 68). Microplate assays were counted in a Microlumat LB96P microplate luminometer (EG&G Berthold). Positive fractions were further subfractionated with an isocratic mobile phase (of 60 or 70% of acetonitrile in water for fractions 5 or 6, respectively). Active fractions were analyzed by LC mass spectrometry (Micromass Instruments). Samples were ionized by positive-ion fast-atom bombardment (FAB), and the molecular ion (M+H) peaks recorded by FAB-MS were further analyzed by tandem mass spectrometry (MS-MS).
Synthesis of HSLs.
The general method described by Chhabra et al. (5) was used to synthesize N-octanoyl-l-homoserine lactone (C8-HSL), N-(3-oxohexanoyl)-l-homoserine lactone (3-oxo-C6-HSL), N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL),and N-dodecanoyl-L-homoserine lactone (C12-HSL). Each compound was purified to homogeneity by semi-preparative HPLC, and its structure was confirmed by mass spectrometry and proton NMR spectroscopy.
RNA extraction and slot blot analysis.
Total RNA was extracted with the RNAeasy total RNA isolation kit (QIAGEN, Courtaboeuf, France) according to the manufacturer's protocol. Samples were treated with DNase (Roche, Bruxelles, Belgium) to remove contaminating DNA. The preparation was heated to 95°C for 5 min and extracted with phenol to remove DNase. The RNA was precipitated with ethanol, collected by centrifugation, dried to near completion, dissolved in an appropriate volume of DEPC double-distilled water, and quantified by spectrophotometry. Slot blot analysis was performed as described by Ausubel et al. (3). Serial dilutions were immobilized on positively charged nylon membrane (Roche), ranging from 1 μg to 15.625 ng of total RNA. The virB5 and l7/l12 probes were labeled by PCR, using 0.1 mM digoxigenin-UTP (Roche) according to the manufacturer's instructions. Probe hybridization, stringency washes, probe detection by an anti-digoxigenin alkaline phosphatase (AP) conjugate, and visualization using the chemiluminescent AP substrate CSPD (Roche) were as directed by the manufacturer (14).
RESULTS
Acyl-HSL activity is present in B. melitensis culture supernatant.
T-streak assays of B. melitensis 16M Nalr against the biosensor strains C. violaceum CV026, E. coli JM109 (pSB401), and E. coli JM109(pSB1075) (Table 1) on 2YT at 37°C failed to detect any acyl-HSL activity. To obtain a concentrated sample of any putative Brucella acyl-HSLs that could be assayed at the recommended temperature of 30°C (31, 68), dichloromethane extracts of spent Brucella culture supernatants (2YT [E16M] and RPMI [ER16M] grown) were prepared and concentrated 10,000-fold in acetonitrile. A twofold end-point dilution assay was performed for each extract with the biosensors E. coli JM109 (pSB401) and E. coli JM109 (pSB1075) by using dilutions of purified 3-oxo-C6-HSL and 3-oxo-C12-HSL, respectively, as positive controls. As a negative control for these assays, an equivalent extract of the noninoculated medium (denoted E2YT and ER, respectively, for 2YT and RPMI 1640). No acyl-HSL activity was detected by E. coli JM109 (pSB401) in extracts from 2YT- or RPMI-cultured B. melitensis 16M Nalr. No acyl-HSL activity was detected by E. coli (pSB1075) in extracts from 2YT; however, a clear activation of the LasR-based biosensor was observed in an extract of RPMI-cultured B. melitensis 16M Nalr (Fig. 1), suggesting that a long-chain acyl-HSL was present. No activation of the biosensors could be seen when incubated with the extract of noninoculated RPMI 1640.
FIG. 1.
RPMI-cultured B. melitensis 16M Nalr cells produce an acyl-HSL activity detected by E. coli (pSB1075). The activation of LasR and the lasI promoter by acyl-HSL activities are shown in twofold dilution series of (left to right) synthetic C12-HSL (5 ng in the first column), synthetic C12-HSL plus solvent extract from 2YT medium (E2YT) (5 ng of C12-HSL plus 5 μl of E2YT [corresponding to 50 ml of medium] in the first column), solvent extraction from RPMI medium (ER; 5 μl [corresponding to 50 ml of medium] in the first column), and solvent extraction from RPMI-cultured B. melitensis 16M Nalr (ER16M; 5 μl [corresponding to 25 ml of medium] in the first column). The expression of lasI′::luxCDABE was measured in relative light units (RLU).
The negative results observed with E16M may be due to inhibitory activities present in the dichloromethane extract, which antagonized activation of the AHL biosensor. To investigate this further, synthetic acyl-HSLs were added to dichloromethane extracts of 2YT, yeast extract (YE), and peptone (Pep) and incubated with biosensors in a twofold dilution series (data not shown). This experiment revealed that extracts of 2YT, YE, and Pep contained compounds that were able to antagonize the acyl-HSL-mediated activation of the AHL biosensors.
B. melitensis produces C12-HSL.
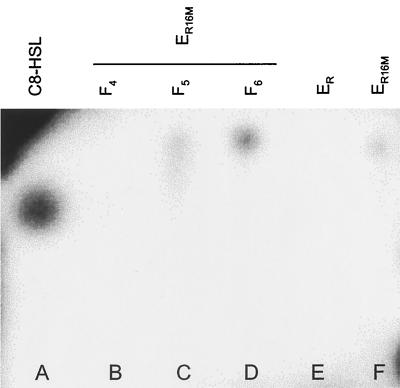
A dichloromethane extract of 18 liters of B. melitensis 16M Nalr-spent RPMI culture supernatant was dried and reconstituted in a final volume of 1.2 ml of acetonitrile. The extract was fractionated using reverse-phase HPLC with a linear gradient of acetonitrile in water. HPLC fractions F5 and F6 activated E. coli (pSB1075) in agar well assays (Fig. 2). Fractionation by TLC on silica plates followed by biosensor overlay (Fig. 3) revealed that each fraction contained an active compound. Moreover, these molecules seem to be different as the active compound present in F5, but not the one in F6, exhibits a characteristic tailing which is commonly observed with 3-oxo-substituted acyl-HSLs. F5 and F6 were therefore subfractionated by HPLC in isocratic conditions (60% for F5 and 70% for F6), and the biologically active subfractions F5.2 and F6.3 were identified. Liquid chromatography coupled to mass spectrometry (LC-MS) analysis of subfraction F6.3 revealed a molecular ion (M+H) peak at m/z 284 and two fragmentation ions at m/z 183 (corresponding to the acyl chain) and 102 (corresponding to the homoserine lactone ring) (Fig. 4). These data indicate that this acyl-HSL is C12-HSL. C12-HSL was synthesized and shown to possess the same HPLC retention time and mass spectrum as the natural product (Fig. 4). For fraction 5.2, no significant spectra containing a peak at m/z 102, indicative of the homoserine lactone ring, was identified.
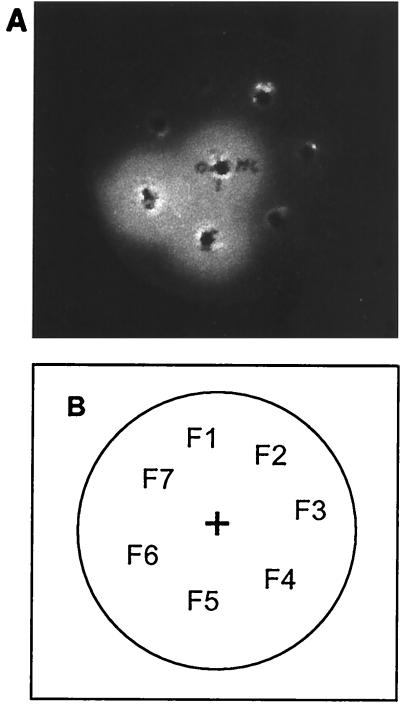
FIG. 2.
HPLC fractionation of the acyl-HSL activity produced in RPMI. (A) Induction of bioluminescence from E. coli (pSB1075) by fractions 5 and 6, collected after HPLC separation of ER16M. For each sample, 5 μl was diluted to 50 μl with LB medium and added to wells cut into the LB agar as described in Materials and Methods. +, positive control (5 ng of 3-oxo-C12-HSL). Fractions 1, 2, 3, 4, and 7 showed no activity. (B) Key to the loading of the plate.
FIG. 3.
TLC fractionation of the acyl-HSL activity produced in RPMI. Normal-phase TLC of the acyl-HSLs present in cell-free supernatant of B. melitensis 16M Nalr (ER16M) was revealed by overlay with E. coli (pSB1075). Samples were spotted on a silica plate and developed with 45% acetone in N-hexane. Lane A, positive control (5 ng of C8-HSL); lanes B to D, fractions 4 to 6 of ER16M, respectively; lane E, negative control (ER); lane F, ER16M.
FIG. 4.
MS of F6.3 identifies C12-HSL. (A) The chemical structure of C12-HSL showing the two fragmentation ions having major peaks in electrospray MS (m/z 102 and m/z 183). (B) Comparison of the MS spectrum of synthetic C12-HSL and of the C12-HSL found in subfraction F6.3 of the culture supernatant extract of B. melitensis 16M Nalr.
C12-HSL has a physiological effect on Brucella.
By definition, QS facilitates the regulation of gene expression in a cell population-dependent manner. In B. suis, the virB operon, encoding a type IV secretion system, is regulated in a growth phase-dependent manner, with virB transcription being reduced in the late exponential phase (3a). We therefore investigated the possibility that it might be the same in B. melitensis and that QS may control the transcription of the virB operon. A B. melitensis 16M Nalr culture was grown, and samples were taken at three different culture stage (lag phase [A590 = 0.05], early exponential phase [A590 = 0.1], and early stationary phase [A590 = 1]), split, and incubated for a further 3 h following the addition of either synthetic C12-HSL to a final concentration of 50 ng ml−1 or an equivalent volume of acetonitrile. After 3 h, the bacterial cells were harvested and total RNA was extracted. Slot blots were probed for the presence of mRNA from the virB operon and from the l7/l12 gene used as housekeeping gene (Fig. 5). First, we observed that the level of virB mRNA was lessened significantly upon reaching the stationary phase, reproducing in B. melitensis what is seen in B. suis. Second, C12-HSL had no detectable effect upon the I7/I12 total mRNA level. In contrast, the amount of virB mRNA from bacteria incubated with 50 ng of C12-HSL ml−1 was substantially reduced when compared with the control bacteria (acetonitrile alone). Addition of C12-HSL thus inhibits virB transcription in B. melitensis and, surprisingly, this appears at every stage of growth assayed. Moreover, the same effect was also observed for cultures of B. suis 1330 cultured with 50 ng of C12-HSL ml−1 (data not shown).
FIG. 5.
Total RNA was extracted from lag (A600= 0.05), early log (A600 = 0.15), and transition (A600 = 1) phase cultures of B. melitensis 16M Nalr in the presence (+) or absence (−) of 50 ng of synthetic C12-HSL ml−1. A twofold dilution series of total RNA for each treatment was immobilized on a positively charged nylon membrane. Blots were hybridized with digoxigenin-labeled virB5 probe (A) and digoxigenin-labeled l7/l12 probe (B) as controls.
DISCUSSION
The evidence presented here demonstrates, for the first time, that acyl-HSLs are produced by an intracellular pathogen. B. melitensis produces the QS signal C12-HSL when cultured at 37°C in vitro in the tissue culture medium RPMI. C12-HSL was identified by mass spectrometry after solvent extraction of spent B. melitensis culture supernatant and fractionation by HPLC. A second active fraction was detected, but the identity of the molecule present could not be determined by mass spectrometry, probably because the amount of this minor signal was too small. Comparison of the migration profiles of this unknown molecule in HPLC and TLC with synthetic acyl-HSL standards suggests that it is most probably 3-oxo-C12-HSL (data not shown). Up to now, we do not know if this second potential HSL has its own specificity or not (67).
In comparison with other bacteria studied, Brucella appears to produce low levels of acyl-HSLs. To obtain sufficient C12-HSL for mass spectrometry, it was necessary to extract 18 liters of spent culture medium (compared with around 4 to 6 liters reported in the literature for other bacteria [e.g., as seen in references 44 and 56]). One explanation for this observation may be found in the hydrophobic nature of C12-HSL. Pearson et al. have shown that the efficiency of extraction depends upon the hydrophobicity of the acyl-HSL (38). For synthetic [3H]3-oxo-C12-HSL incubated with a P. aeruginosa culture, only 75% of the signal is recovered by the extraction process, with 25% remaining trapped in bacterial membranes. C12-HSL is more hydrophobic than 3-oxo-C12-HSL, so we expected that in our extractions of the Brucella spent supernatant that less than 75% of the total production would have been recovered.
The intracellular lifestyle of Brucella may provide a second explanation for the production of low levels of acyl-HSL. Brucella cells survive intracellularly in mammalian host cells but do not begin to replicate until they have modified intracellular trafficking to produce the replication vacuole. At this point and depending on the type of cell infected, some heterogeneity of behavior has been observed. In placental trophoblasts, for example, the bacteria multiply within membrane-bound intracellular compartments until the infected cell lyses. In chronic infection, however, Brucella cells survive in macrophages and can be seen in low numbers. In either case, however, Brucella will be found within a confined compartment and any C12-HSL produced by Brucella will accumulate, especially given the slow diffusion of hydrophobic acyl-HSLs across membranes, leading to the activation of QS at a lower cell density than in an open environment (38). Given that Brucella cells are unlikely to encounter any other sources of C12-HSL within their specialized intracellular niche, it is probable that they have evolved a system sensitive to low levels of signal.
An alternative third explanation for low levels of acyl-HSL production in vitro is a possible regulation mechanism controlling the HSL synthesis. A mechanism that could rely on the presence of a typical environment or a key host cell-derived signal to activate acyl-HSL production. This may be particularly relevant given the influence of the host cell-derived signals (like opines and flavonoids) on QS in other alpha subgroup proteobacteria (Agrobacterium and Rhizobium, respectively) (8, 9, 39) and the possibility that C12-HSL may be produced for both its effects upon the bacterial gene regulation and its effects upon the host organism.
Extracellular pathogens, such as P. aeruginosa, Erwinia carotovora, and Aeromonas hydrophyla, use QS to control the expression of virulence factors. This was explained by the necessity to prevent an early release of these factors that would elicit an inflammatory or immune response until the population density is high enough to cope successfully with this host response (21, 36, 40, 56). We have found that QS could be linked to Brucella virulence by downregulating expression of the virB operon. The transcription of the virB genes is reduced in the late exponential phase. Slot blot experiments probing for virB mRNA reveal that this phenomenon can be mimicked in lag and early exponential phase by the addition of exogenous C12-HSL but also enhanced in the transition phase of growth. This effect is observed in cultures of both B. melitensis 16M Nalr and B. suis, suggesting a common role for C12-HSL in gene regulation in both species. The VirB system has been shown to be essential for virulence in both in vitro and in vivo infection models, possibly playing a role in the establishment of the replication niche (6, 11, 45). This control of the expression of virB by QS suggests that expression of VirB is required only in the early stages of the infection and that it is perhaps detrimental at later stages. This hypothesis is supported by our observation that the presence of the virB operon on a pBBR1MCS derivative (10 copies per cell) complements the defect in intracellular replication of Brucella virB mutants only in the early stages of the infection. Furthermore, the presence of this plasmid inhibits intracellular multiplication of wild-type B. suis between 24 and 48 h postinfection (34).
The regulation of gene expression by QS is most commonly described as a process of activation. However, negative regulation has also been described, e.g., for Burkholderia cepacia, where QS induces protease production but also represses siderophore production (28), and Yersinia pseudotuberculosis, where QS appears to repress flagellin production (and hence flagellar-mediated motility) (2). Analysis of the QS regulon in Brucella may therefore identify genes positively and/or negatively regulated and will give important information regarding the factors required during different phases of the infection process.
Recently, Sieira et al. (51) described the virB operon in B. abortus 2308 and reported that expression is induced in the stationary phase by monitoring β-galactosidase activity in a virB10::lacZ fusion strain. This result, shown for B. abortus, is in contradiction to the data we present here and elsewhere for B. melitensis and B. suis (Boschiroli et al., submitted). However, the experimental conditions, and hence the species, used by Sieira et al. are different from those described in this paper. B. abortus cells were cultured in tryptone soy broth, a rich medium similar to 2YT and not to RPMI or E medium. We suggest that compounds present in tryptone soy broth may inhibit the QS regulation of virB expression in Brucella. Indeed, we have not been able to demonstrate any significant effect of C12-HSL upon virB transcription in rich medium (data not shown).
The nature of the inhibitory compound is not known, however; Holden et al. (17) demonstrated the presence of small cyclic dipeptides or diketopiperazines (DKPs) in Pseudomonas culture supernatant that are able to activate or to antagonize QS biosensor systems. They showed that several DKPs are able to antagonize the induction of bioluminescence in E. coli (pSB401) by 3-oxo-C6-HSL without interfering with light emission from E. coli harboring constitutively expressed lux genes. Moreover, DKPs have been isolated from protein digests used as media components, and so the inhibitory effects observed with 2YT could be attributed to these molecules.
The immunosuppressive effect of 3-oxo-C12-HSL upon the host immune system observed by Telford et al. (60) prejudices against antibacterial action and so favors the bacteria. Additionally, the vasorelaxant activity of 3-oxo-C12-HSL observed by Lawrence et al. (26) may contribute to the ability of the bacteria to maintain the supply of key nutrients to the site of infection by increasing local blood flow. This acyl-HSL, produced by P. aeruginosa, can also modulate the expression of membrane receptors of human tracheal gland cells, aggravating Pseudomonas infections in cystic fibrosis patients (47). Given the chemical proximity between 3-oxo-C12-HSL and those produced by Brucella, a possible effect of C12-HSL on the immune system should not be ignored, especially given the biology of Brucella and its ability to deal with cellular actors of the immune system. Indeed, 3-oxo-C12-HSL has been shown to inhibit interleukin-12 and tumor necrosis factor alpha production, thus creating a situation potentially leading to a TH2 immune response (60). As an intracellular pathogen, Brucella has been shown to be more susceptible to a TH1-oriented response. For instance, depletion of IL-12 leads to an exacerbated infection by Brucella (69). Taken together, the possibility that C12-HSL and 3-oxo-C12-HSL could have a similar effect on cytokine production would obviously benefit Brucella.
We plan to extend our investigation of the role of QS in Brucella infections. We benefit now from the availability of the B. melitensis genome sequence for the search of the luxI/luxR homologs. The deletion of these genes will allow us to evaluate their impact upon the virulence of Brucella. Moreover, it will be interesting to identify other genes under the control of QS in Brucella.
Acknowledgments
We are grateful to J.-M. Verger (Laboratoire de Pathologie Infectieuse et d'Immunologie, Institut National de Recherche Agronomique, Nouzilly, France) for the B. melitensis 16M Nalr nalidixic acid-resistant strain.
Bernard Taminiau holds a specialization grant from the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA). This work was supported in part by a grant (to P. Williams) from the Biotechnology and Biological Sciences Research Council U.K. and in part by the Commission of the European Communities, contract no. QLK2-CT-1999-00014 (Anne Tibor), which are gratefully acknowledged.
Editor: V. J. DiRita
REFERENCES
- 1.Allen, C. A., L. G. Adams, and T. A. Ficht. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, S., J. P. Throup, G. S. Stewart, and P. Williams. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Short protocols in molecular biology, 3rd ed. John Wiley & Sons, Inc., New York, N.Y.
- 3a.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O’Callagghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 92:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns, D. L. 1999. Biochemistry of type IV secretion. Curr. Opin. Microbiol. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra, S. R., P. Stead, N. J. Bainton, G. P. Salmond, G. S. Stewart, P. Williams, and B. W. Bycroft. 1993. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J. Antibiot. (Tokyo) 46:441-454. [DOI] [PubMed] [Google Scholar]
- 6.Coers, J., C. Monahan, and C. R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell. Biol. 1:451-453. [DOI] [PubMed] [Google Scholar]
- 7.Cotter, P. A., and J. F. Miller. 1998. In vivo and ex vivo regulation of bacterial virulence gene expression. Curr. Opin. Microbiol. 1:17-26. [DOI] [PubMed] [Google Scholar]
- 8.Cubo, M. T., A. Economou, G. Murphy, A. W. Johnston, and J. A. Downie. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui, Y., A. Chatterjee, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 177:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 11.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 12.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunny, G. M., and S. C. Winans. 1999. Bacterial life: neither lonely nor boring, p. 1-5. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 14.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 2000. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 68:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density- responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfroid, F., B. Taminiau, I. Danese, P. Denoel, A. Tibor, V. Weynants, A. Cloeckaert, J. Godfroid, and J. J. Letesson. 1998. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect. Immun. 66:5485-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holden, M. T., S. Ram Chhabra, R. de Nys, P. Stead, N. J. Bainton, P. J. Hill, M. Manefield, N. Kumar, M. Labatte, D. England, S. Rice, M. Givskov, G. P. Salmond, G. S. Stewart, B. W. Bycroft, S. Kjelleberg, and P. Williams. 1999. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol. 33:1254-1266. [DOI] [PubMed] [Google Scholar]
- 18.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inon de Iannino, N., G. Briones, M. Tolmasky, and R. A. Ugalde. 1998. Molecular cloning and characterization of cgs, the Brucella abortus cyclic beta(1-2) glucan synthetase gene: genetic complementation of Rhizobium meliloti ndvB and Agrobacterium tumefaciens chvB mutants. J. Bacteriol. 180:4392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, Y., M. Camara, S. R. Chhabra, K. R. Hardie, B. W. Bycroft, A. Lazdunski, G. P. Salmond, G. S. Stewart, and P. Williams. 1998. In vitro biosynthesis of the Pseudomonas aeruginosa quorum-sensing signal molecule N-butanoyl-L-homoserine lactone. Mol. Microbiol. 28:193-203. [DOI] [PubMed] [Google Scholar]
- 21.Jones, S., B. Yu, N. J. Bainton, M. Birdsall, B. W. Bycroft, S. R. Chhabra, A. J. Cox, P. Golby, P. J. Reeves, S. Stephens, et al. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler, S., J. Teyssier, A. Cloeckaert, B. Rouot, and J. P. Liautard. 1996. Participation of the molecular chaperone DnaK in intracellular growth of Brucella suis within U937-derived phagocytes. Mol. Microbiol. 20:701-712. [DOI] [PubMed] [Google Scholar]
- 24.Kulakov, Y. K., P. G. Guigue-Talet, M. R. Ramuz, and D. O'Callaghan. 1997. Response of Brucella suis 1330 and B. canis RM6/66 to growth at acid pH and induction of an adaptive acid tolerance response. Res. Microbiol. 148:145-151. [DOI] [PubMed] [Google Scholar]
- 25.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence, R. N., W. R. Dunn, B. Bycroft, M. Camara, S. R. Chhabra, P. Williams, and V. G. Wilson. 1999. The Pseudomonas aeruginosa quorum-sensing signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone, inhibits porcine arterial smooth muscle contraction. Br. J. Pharmacol. 128: 845-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lestrate, P., R. M. Delrue, I. Danese, C. Didembourg, B. Taminiau, P. Mertens, X. De Bolle, A. Tibor, C. M. Tang, and J. J. Letesson. 2000. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol. Microbiol. 38:543-551. [DOI] [PubMed] [Google Scholar]
- 28.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dye, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 30.Lynch, M. J., S. Swift, D. F. Kirke, C. E. R. Dodd, C. W. Keevil, G. S. A. B. Stewart, and P. Williams. 1999. Investigation of quorum sensing in Aeromonas hydrophila biofilms formed on stainless steel, p. 209-221. In J. Wimpenny, P. Gilbert, J. Walker, M. Brading, and R. Bayston (ed.), Biofilms: the good, the bad and the ugly. BioLine, Cardiff, United Kingdom.
- 31.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143(Pt. 12):3703-3711. [DOI] [PubMed] [Google Scholar]
- 32.McQuiston, J. R., R. Vemulapalli, T. J. Inzana, G. G. Schurig, N. Sriranganathan, D. Fritzinger, T. L. Hadfield, R. A. Warren, N. Snellings, D. Hoover, S. M. Halling, and S. M. Boyle. 1999. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect. Immun. 67:3830-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.More, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 34.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 35.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to- cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 37.Pearson, J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piper, K. R., S. Beck Von Bodman, I. Hwang, and S. K. Farrand. 1999. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobacterium. Mol. Microbiol. 32:1077-1089. [DOI] [PubMed] [Google Scholar]
- 40.Pirhonen, M., D. Flego, R. Heikinheimo, and E. T. Palva. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 12:2467-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson, G. T., and R. Roop. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690-700. [DOI] [PubMed] [Google Scholar]
- 44.Rodelas, B., J. K. Lithgow, F. Wisniewski-Dye, A. Hardman, A. Wilkinson, A. Economou, P. Williams, and J. A. Downie. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 181:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 46.Rumbaugh, K. P., J. A. Griswold, B. H. Iglewski, and A. N. Hamood. 1999. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleh, A., C. Figarella, W. Kammouni, S. Marchand-Pinatel, A. Lazdunski, A. Tubul, P. Brun, and M. D. Merten. 1999. Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect. Immun. 67:5076-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer, A. L., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 93:9505-9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shadel, G. S., and T. O. Baldwin. 1992. Positive autoregulation of the Vibrio fischeri luxR gene. LuxR and autoinducer activate cAMP-catabolite gene activator protein complex-independent and -dependent luxR transcription. J. Biol. Chem. 267:7696-7702. [PubMed] [Google Scholar]
- 50.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 53.Smith, L. D., and T. A. Ficht. 1990. Pathogenesis of Brucella. Crit. Rev. Microbiol. 17:209-230. [DOI] [PubMed] [Google Scholar]
- 54.Sola-Landa, A., J. Pizarro-Cerda, M. J. Grillo, E. Moreno, I. Moriyon, J. M. Blasco, J. P. Gorvel, and I. Lopez-Goni. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 55.Stevens, A. M., and E. P. Greenberg. 1997. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J. Bacteriol. 179:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swift, S., A. V. Karlyshev, L. Fish, E. L. Durant, M. K. Winson, S. R. Chhabra, P. Williams, S. Macintyre, and G. S. Stewart. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 179:5271-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swift, S., M. J. Lynch, L. Fish, D. F. Kirke, J. M. Tomas, G. S. Stewart, and P. Williams. 1999. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect. Immun. 67:5192-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swift, S., G. S. Stewart, and P. Williams. 1996. The inner workings of a quorum sensing signal generator. Trends Microbiol. 4:463-466. [DOI] [PubMed] [Google Scholar]
- 59.Swift, S., P. Williams, and G. S. A. B. Stewart. 1999. N-acylhomoserine lactones and quorum sensing in Proteobacteria, p. 291-314. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 60.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3- oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Val, D. L., and J. E. Cronan, Jr. 1998. In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J. Bacteriol. 180:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verger, J. M., M. Grayon, E. Chaslus-Dancla, M. Meurisse, and J. P. Lafont. 1993. Conjugative transfer and in vitro/in vivo stability of the broad-host-range IncP R751 plasmid in Brucella spp. Plasmid 29:142-146. [DOI] [PubMed] [Google Scholar]
- 63.Visick, K. L., J. Foster, J. Doino, M. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Bodman, S. B., D. R. Majerczak, and D. L. Coplin. 1998. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA 95:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welch, M., D. E. Todd, N. A. Whitehead, S. J. McGowan, B. W. Bycroft, and G. P. Salmond. 2000. N-acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 19:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams, P., M. Camara, A. Hardman, S. Swift, D. Milton, V. J. Hope, K. Winzer, B. Middleton, D. I. Pritchard, and B. W. Bycroft. 2000. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355:667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, B. W. Bycroft, et al. 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]
- 69.Zhan, Y., A. Kelso, and C. Cheers. 1995. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect. Immun. 63:969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, J., and S. C. Winans. 1999. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc. Natl. Acad. Sci. USA 96:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]