Abstract
Stress-activated protein kinases (SAPKs), consisting of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK), are activated upon various environmental stimuli, including viral infections. Cellular survival and death signaling events following coxsackievirus B3 (CVB3) infection have been studied in relationship to viral replication, but the role of SAPKs has not been scrutinized. In this study, we found that the phosphorylation of JNK1/2 and p38 MAPK was increased during active replication of CVB3 and that their phosphorylation was independent of CVB3-induced caspase activation or production of reactive oxygen species. The roles of these kinases in CVB3 infection were further evaluated using specific inhibitors: SP600125 for JNK1/2 and SB203580 for p38 MAPK. JNK1/2 inhibitors reduced CVB3-induced phosphorylation of activating transcription factor 2, and the p38 MAPK inhibitor reduced CVB3-induced phosphorylation of heat shock protein 27. Although inhibition of these kinases by specific inhibitors did not affect CVB3 viral protein synthesis, inhibition of p38 MAPK but not of JNK1/2 resulted in significant reduction of viral progeny release, suppression of CVB3-induced cell death, and blockage of CVB3-induced caspase-3 activation in infected cells. We conclude that SAPK pathways play critical roles in the life cycle of CVB3, particularly in viral progeny release.
Coxsackievirus B3 (CVB3), the primary human pathogen of viral myocarditis, is a member of the Enterovirus genus of the family Picornaviridae. CVB3 contains a single copy of positive-strand RNA genome which was decoded in the 1980s (21). In the last 2 decades, enormous work has been undertaken to dissect the molecular mechanisms underlying the pathogenesis of CVB3-induced diseases. From this work, the understanding of the transcriptional and translational control of virus replication, the innate and acquired immunity of myocarditis, and the survival and death signaling of CVB3 in both cell culture systems and animal models has been further refined (2, 7, 16, 27, 32). Studies from our laboratory and from others have shown that CVB3 infection results in activation of several cellular survival signaling pathways, including p56lck nonreceptor tyrosine kinase, extracellular signal-regulated kinase (ERK), and phosphoinositide-3 kinase (PI3K)/Akt (13, 15, 26, 29, 35). Activation of some of these kinases could delay the onset of CVB3-induced apoptosis of infected cells and facilitate replication of CVB3 (13, 15, 26, 29, 35). CVB3 infection also results in cytochrome c release and caspase-3 cleavage, and this activation of cell death signaling pathways may facilitate viral progeny release (5, 6, 13, 30, 41, 52). In addition, viral protein 2B has been found to suppress apoptotic host cell responses by manipulating intracellular calcium homeostasis (14, 47, 48). However, the role of stress-activated pathways in CVB3-induced pathogenesis has not been well elucidated.
Stress-activated protein kinases (SAPKs), which are members of the mitogen-activated protein kinase (MAPK) family, include c-Jun N-terminal kinase (JNK) and p38 MAPK (8, 22, 49). Similarly to other members of this family, JNK and p38 MAPK are activated by various stimuli, including stress, UV irradiation, and proinflammatory cytokines, through a MAPK activation module which consists of a MAPK kinase kinase (MEKK), a MAPK kinase (MEK), and MAPK. Recent studies have shown that a number of virus infections lead to activation of JNK1/2 and p38 MAPK, and activation of these SAPKs is required for viral replication and viral progeny release (11, 19, 31, 38, 42). However, the mechanisms leading to the activation of these SAPKs and the effects of SAPK activation on replication differ among different viruses.
In this study, we show for the first time that the phosphorylation of both JNK1/2 and p38 is increased during CVB3 replication. However, only inhibition of p38 MAPK, and not inhibition of JNK1/2, reduces CVB3-induced cell death, caspase-3 activation, and CVB3 viral progeny release, while having little effect on viral protein expression.
MATERIALS AND METHODS
Cell culture, virus, and materials.
HeLa cells (American Type Culture Collection) were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated newborn calf serum (Invitrogen). CVB3 (Kandolf strain) was propagated in HeLa cells and stored at −80°C. Virus titers were routinely determined by plaque assays as described below.
SP600125, SB203580, and the JNK peptide inhibitor 1 were purchased from Calbiochem. The general caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp- fluoromethylketone (zVAD.fmk) was obtained from BD Biosciences. The monoclonal anti-β-actin antibody and antioxidant N-acetyl-l-cysteine (NAC) were purchased from Sigma Chemical Company. The monoclonal anti-VP1 antibody was purchased from DakoCytomation. The polyclonal anti-phospho-p38 MAPK was purchased from Cell Signaling. The monoclonal anti-hsp27, anti-phospho-JNK, and anti-phospho-ATF-2 antibodies, polyclonal anti-phospho-hsp27 antibody, and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology.
Virus infection.
HeLa cells were grown in complete medium (DMEM supplemented with 10% newborn calf serum) to 70 to 80% confluence prior to infection. HeLa cells were then infected with CVB3 at a multiplicity of infection (MOI) of 10, unless otherwise indicated, or sham infected with phosphate-buffered saline (PBS) for 1 h in serum-free DMEM. Cells were then washed with PBS and cultured in serum-free DMEM for the indicated periods of time. For inhibitor experiments, HeLa cells were preincubated with inhibitors for 30 min and then infected with CVB3. One hour postinfection, cells were washed with PBS twice and then incubated with fresh DMEM containing various concentrations of compounds.
Western blot analysis.
Cell lysates were prepared as described previously (29). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. Membranes were blocked for 1 h with nonfat dry milk solution (3% in PBS) containing 0.1% Tween 20. Blots were then incubated with the primary antibody for 1 h, followed by incubation for an additional hour with the secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
Cell viability assay.
A modified 3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) assay (Promega), which measures mitochondrial function, was used to detect cell viability. HeLa cells were grown in 96-well plates and serum starved for 24 h. Following CVB3 infection (MOI = 1), the culture medium was replaced with serum-free DMEM with or without inhibitors. Twenty hours postinfection, cells were incubated in MTS solution for 2 h, and absorbance was measured with an enzyme-linked immunosorbent assay plate reader (490 nm). MTS assays were performed in triplicate.
Caspase-3 activity assay.
Caspase-3 activity assay was measured according to the manufacturer's suggestion (R&D Systems). HeLa cells were preincubated with various inhibitors for 30 min and then infected with CVB3 (MOI = 5) or sham infected with PBS. Seven hours postinfection, cell lysates were harvested and subjected to caspase-3 activity assay by use of a fluorogenic substrate.
Plaque assay.
CVB3 titers in cell supernatants were determined on monolayers of HeLa cells by an agar overlay plaque assay in triplicate as described previously (1). Briefly, samples were serially diluted 10-fold and overlaid on 90- to 95%-confluent monolayers of HeLa cells in six-well plates and incubated for 1 h. Medium was aspirated, HeLa cells were washed with PBS twice, and 2 ml of complete DMEM containing 0.75% agar was overlaid onto each well. Cells were incubated at 37°C for 72 h, fixed with Carnoy's fixative (75% ethanol-25% acetic acid) for 30 min, and stained with 1% crystal violet. Plaques were counted, and viral concentrations were calculated as PFU per milliliter.
Statistical analysis.
Two-way analysis of variance with multiple comparisons and paired Student's t tests were performed. Values shown are the means ± standard deviations (SD). A P value of < 0.05 was considered significant.
RESULTS
Activation of SAPKs during CVB3 replication.
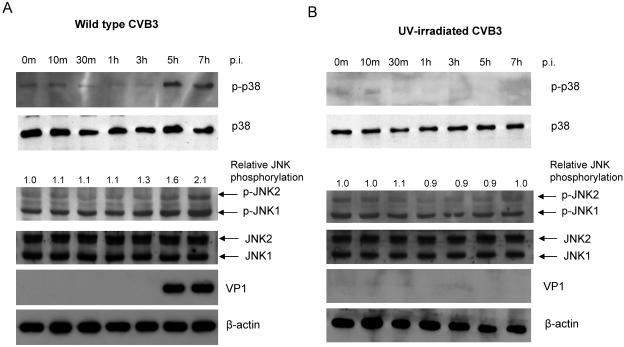
It has been reported that SAPKs are phosphorylated during replication of various viruses, including human immunodeficiency virus (11, 42), Sindbis virus (34), and encephalomyocarditis virus (EMCV) (19). In the current study, we investigated whether infection with CVB3 could cause activation of JNK and p38 MAPK, two key members of the SAPKs. As shown in Fig. 1A, we found that p38 MAPK was phosphorylated beginning at approximately 5 hours postinfection, during the active virus replication cycle. There was also an increase in the phosphorylation levels of JNK1/2 at the same time, although the increase in phosphorylation is much less dramatic for JNK than it is for p38. The increased phosphorylation of p38 MAPK and JNK1/2 was concurrent with expression of viral capsid protein VP1 in infected cells.
FIG. 1.
Time course for CVB3 stimulation and phosphorylation of JNK1/2 and p38 MAPK. HeLa cells were infected with wild-type (A) or UV-irradiated (B) CVB3 at MOIs of 10, and cell lysates were collected at the indicated times. Aliquots of cell lysates were examined for phosphorylation statuses of JNK1/2 and p38 MAPK and for expression level of CVB3 viral capsid protein VP1. Total JNK1/2 and β-actin were probed as loading controls. Changes of JNK1/2 phosphorylation (n-fold) were quantitated by densitometric analysis using ImageJ (National Institutes of Health, version 1.32j) and normalized to total JNK. Phosphorylation of JNK1/2 in sham-infected cells was arbitrarily set to 1.0. Similar results were obtained in three independent experiments. p-, phospho-.
To further explore possible mechanisms of activation of JNK1/2 and p38 MAPK during CVB3 replication, we utilized UV-irradiated CVB3 that was capable of virus receptor engagement but unable to replicate (29). As shown in Fig. 1B, there were no significant changes in the phosphorylation levels of p38 MAPK and JNK1/2 in cells infected with UV-irradiated CVB3, suggesting that phosphorylation of p38 MAPK and JNK1/2 was associated with CVB3 replication.
Activation of p38 MAPK and JNK1/2 is independent of CVB3-induced oxidative stress or caspase activation.
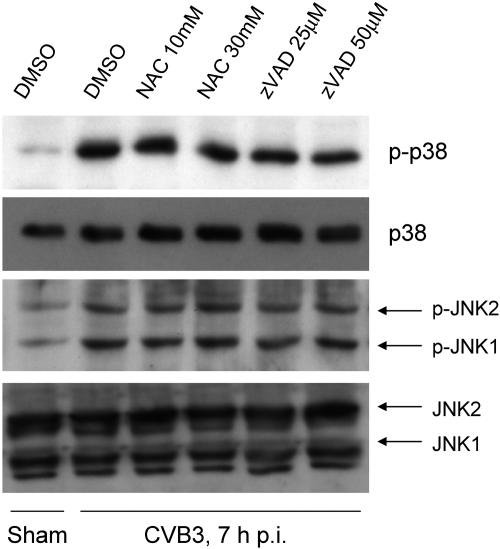
We have previously shown that there is an increased reactive oxygen species (ROS) generation during CVB3 replication, and ROS production could be alleviated by treatment with antioxidant NAC or general caspase inhibitor zVAD.fmk (44). Since oxidative stress has been reported to mediate p38 MAPK and JNK1/2 activation under many different conditions (20, 33, 37), we sought to investigate whether oxidative stress plays causative roles in activation of p38 MAPK and JNK1/2 during CVB3 replication. We found that oxidative stress alleviation by NAC or caspase inhibition by zVAD.fmk did not prevent CVB3-induced p38 MAPK and JNK1/2 activation (Fig. 2), suggesting that p38 MAPK and JNK1/2 activation was unlikely to be caused by CVB3-induced oxidative stress and caspase activation.
FIG. 2.
Activation of p38 MAPK and JNK is independent of oxidative stress and caspase-3 activation. HeLa cells were preincubated with a general caspase inhibitor (zVAD.fmk) or an antioxidant NAC for 30 min and then infected with CVB3 (MOI = 10). One hour later, cells were washed with PBS and replenished with serum-free medium containing fresh inhibitor or antioxidant. Seven hours later, cell lysates were examined for phosphorylation of p38 MAPK and JNK and expression of p38 MAPK and JNK. β-actin was probed as the loading control. Similar results were obtained in two independent experiments. DMSO, dimethyl sulfoxide. p-, phospho-.
Effects of inhibition of SAPKs on CVB3 life cycle.
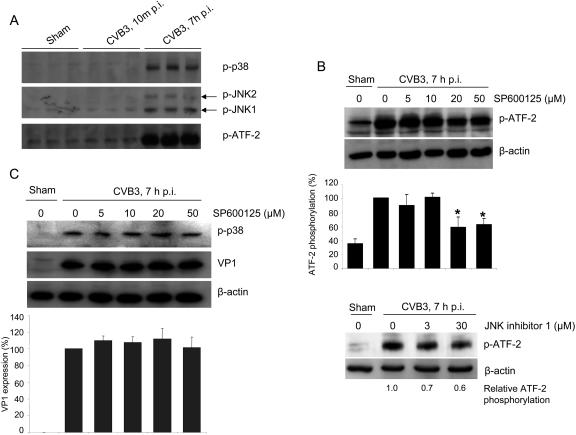
We next investigated the roles of the activation of JNK1/2 and p38 MAPK in CVB3 viral protein synthesis by using specific inhibitors: SP600125 (for JNK1/2) (4) and SB203580 (for p38 MAPK) (12). We chose to use activating transcription factor 2 (ATF-2) and heat shock protein 27 (hsp27), which are downstream substrates of JNK1/2 and p38 MAPK pathways (18, 24, 25, 28, 39, 45), to evaluate optimal doses of these inhibitors. We found that there was a significant increase in the phosphorylation status of ATF-2 during CVB3 replication but not following CVB3 receptor engagement (Fig. 3A). The JNK1/2 inhibitor SP600125 reduced CVB3-induced phosphorylation of ATF-2 by up to 50% at concentrations of 20 μM and 50 μM (Fig. 3B). To confirm that activation of JNK1/2 following CVB3 infection is responsible for ATF-2 activation, we used a peptide inhibitor of JNK (JNK inhibitor 1), and found that it was also able to reduce CVB3-induced ATF-2 phosphorylation (Fig. 3B).
FIG. 3.
Inhibition of JNK1/2 does not affect CVB3 viral protein synthesis. (A) HeLa cells were sham infected with PBS or infected with CVB3 (MOI = 10) for 10 min or for 7 h, respectively. Cell lysates were immunoblotted with anti-phospho-p38, anti-phospho-JNK, and anti-phospho-ATF-2 antibodies. (B) HeLa cells were preincubated with JNK1/2 inhibitors (top panel, SP600125; bottom panel, peptide JNK inhibitor 1) for 30 min and then infected with CVB3 (MOI = 10) for 6 h. Western blotting was performed using an anti-phospho-ATF-2 antibody. Changes in ATF-2 phosphorylation were quantitated by densitometric analysis using ImageJ (National Institutes of Health, version 1.32j) and normalized to control levels (CVB3-infected cells without inhibitors) arbitrarily set to 100%. Results are means ± SD (n = 3). *, P < 0.05 compared to nontreated cells. (C). HeLa cells were infected in the absence or presence of SP600125 as described above. Cell lysates were harvested to examine CVB3 capsid protein VP1 expression and p38 phosphorylation. β-actin was probed as the loading control. Results are means ± SD (n = 3). p-, phospho-.
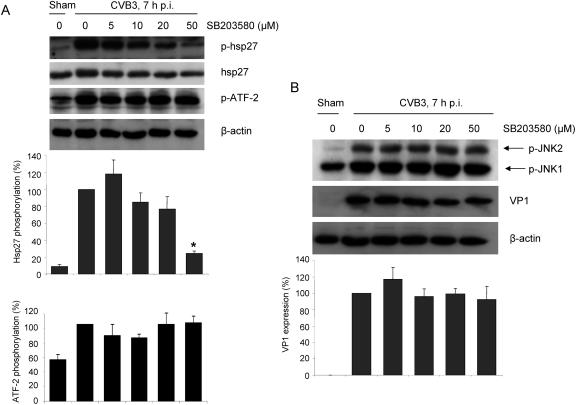
We also found that CVB3 infection led to a marked increase in phosphorylation levels of hsp27, and there was a dose-dependent decrease of hsp27 phosphorylation upon treatment with p38 MAPK inhibitor (Fig. 4A). Phosphorylation levels of hsp27 in infected cells decreased to approximately 30% of the peak level when treated with 50 μM p38 MAPK inhibitor. However, inhibition of p38 MAPK did not reduce CVB3-induced ATF-2 phosphorylation (Fig. 4A).
FIG. 4.
Inhibition of p38 MAPK does not affect CVB3 viral protein synthesis. (A) HeLa cells were preincubated with p38 inhibitor SB203580 for 30 min and then infected with CVB3 (MOI = 10) for 6 h. Cell lysates were collected and immunoblotted with antibodies against phospho-hsp27 (p-hsp27) and phospho-ATF-2. hsp27 was probed as the loading control for p-hsp27, and β-actin was probed as the loading control for p-ATF-2. Phosphorylation of hsp27 and ATF-2 was quantitated by densitometric analysis using ImageJ (National Institutes of Health, version 1.32j) and normalized to control levels (CVB3-infected cells without inhibitor) arbitrarily set to 100%. Results are means ± SD (n = 3). *, P < 0.05 compared to nontreated cells. (B) HeLa cells were infected in the absence or presence of the p38 inhibitor SB203580 as described above. Cell lysates were collected to examine the expression of viral capsid protein VP1 and the phosphorylation of JNK1/2. β-actin was probed as the loading control. Results are means ± SD (n = 3). p-, phospho-.
We then examined the CVB3-induced phosphorylation status of JNK1/2 and p38 MAPK and the expression of viral capsid protein VP1 upon specific inhibitor treatment. As shown in Fig. 3C and 4B, neither did SP600125 interfere with CVB3-induced activation of p38 MAPK, nor did SB203580 interfere with CVB3-induced activation of JNK1/2, suggesting that these two pathways were independently regulated during the virus life cycle. We also did not detect any significant inhibition of viral protein synthesis by using these inhibitors (Fig. 3C and 4B), suggesting that SAPK pathways play roles distinct from those of the previously characterized ERK1/2 and PI3K/Akt pathways (13, 15, 35).
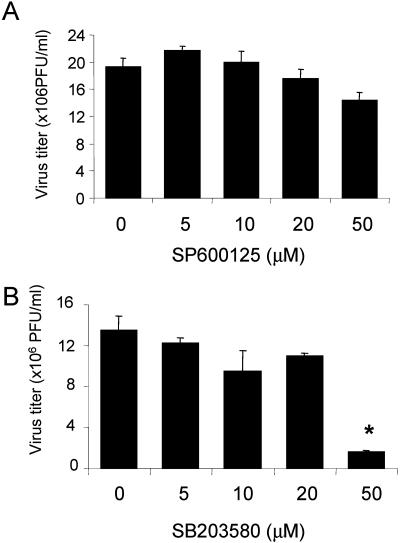
The last step of the life cycle of an enterovirus is to release viral progeny into the medium (5). Our previous studies have shown that apoptosis pathways play an important role in regulating viral progeny release, as evidenced by a decrease of viral progeny release in cells treated with general caspase inhibitor (6). Since one of the best-characterized roles of SAPKs is to respond to environmental stress and deliver signals for apoptosis when there is an irrevocable damage occurring, we further investigated the roles of SAPK pathways in viral progeny release. We found that inhibition of p38 MAPK but not of JNK was associated with a significant reduction of viral progeny release into the medium, as assayed by plaque assay (Fig. 5). High-dose (50μM) SB203580 treatment resulted in a nearly eightfold reduction of viral progeny release (Fig. 5B), whereas SP600125 treatment did not significantly reduce viral progeny release (Fig. 5A). Taken together, these data suggest that the p38 MAPK pathway, but not the JNK pathway, plays a pivotal role in regulating viral progeny release.
FIG. 5.
Inhibition of p38 MAPK, but not of JNK1/2, reduces CVB3 viral progeny release. HeLa cells were preincubated with the JNK inhibitor SP600125 (A) or the p38 inhibitor SB203580 (B) for 30 min and then infected with CVB3 (MOI = 1). Twenty four hours postinfection, cell supernatants were collected, and virus titers were determined by plaque assays on HeLa cell monolayers. Values are means ± SD from three independent experiments. *, P < 0.05 compared to nontreated cells.
Effects of inhibition of SAPKs on cell viability of infected cells.
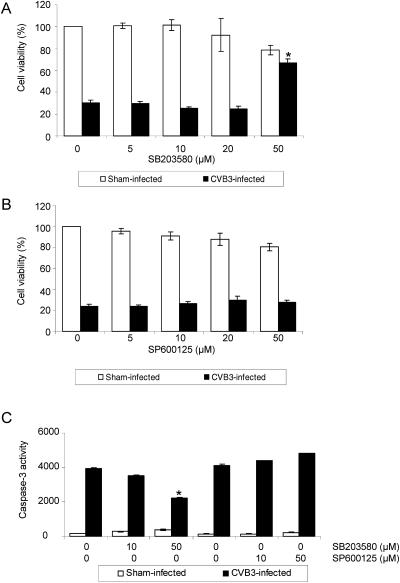
CVB3 infection has been shown to cause cytopathic effects in infected cells and eventually cause cell death by activating intracellular death signaling pathways (5, 13). It has also been shown that JNK1/2 and p38 MAPK activation is often linked to apoptotic signals (9, 49, 51). We thus investigated whether inhibition of JNK1/2 or p38 MAPK could reduce cell death in CVB3-infected cells. As shown in Fig. 6A, inhibition of p38 MAPK by SB203580 significantly improved viability by more than twofold for infected cells at a dose of 50 μM, although the inhibitor treatment alone reduced cell viability by approximately 20%. However, inhibition of JNK1/2 by SP600125 did not prevent the death of infected cells (Fig. 6B). To further explore whether activation of p38 MAPK and JNK1/2 plays any roles in CVB3-induced apoptosis of infected cells, we measured caspase-3 activity in these cells. We found that inhibition of p38 MAPK by SB203580, but not inhibition of JNK1/2 by SP600125, significantly reduced caspase-3 activity in infected cells (Fig. 6C), suggesting that reduction of apoptosis upon inhibition of p38 MAPK contributed to decreased CVB3 progeny release and prolonged cell viability of infected cells. Taken together, our data suggest that activation of p38 MAPK following active viral replication of CVB3 plays an important role in mediating CVB3-induced cell death in order to efficiently release viral progeny into the medium for further infection.
FIG. 6.
Inhibition of p38 MAPK, but not of JNK, reduces cell death of CVB3-infected cells. (A and B) HeLa cells were preincubated with inhibitors of p38 MAPK (panel A) or JNK1/2 (panel B) for 30 min and then infected with CVB3 (MOI = 1). Twenty hours postinfection, cell viabilities of infected cells were measured by an MTS assay. Values are means ± SD from three independent experiments. The cell viability of sham-infected cells was arbitrarily set to 100%. *, P < 0.05 compared to nontreated cells. (C) HeLa cells were treated with inhibitors and infected with CVB3 (MOI = 5) as described above. Seven hours postinfection, caspase-3 activities were measured using a fluorogenic substrate. Values are means ± SD from three independent experiments. *, P < 0.01 compared to nontreated cells.
DISCUSSION
Viral infections are known to activate various cellular signaling pathways to facilitate replication. We and others have shown that CVB3 replication results in activation of cell survival pathways mediated by activation of ERK1/2 (13, 29, 35) and PI3K/AKT (15) and in activation of cell death pathways mediated by cytochrome c release and caspase-3 cleavage (5, 13, 30, 52). CVB3 replication also induces prominent production of ROS following caspase cleavage, which might contribute to activation of these cellular pathways, which are required for virus replication and viral progeny release (44). Activation of SAPKs during replication of a variety of viruses has been reported; however, their roles in CVB3 infection remain unclear.
In this study, we found that both JNK1/2 and p38 MAPK were independently activated during the course of CVB3 infection in HeLa cells, and activation of both JNK1/2 and p38 MAPK required active replication of CVB3. The independent activation of JNK1/2 and p38 MAPK, as well as that of ERK1/2, which we and others have reported previously (13, 29, 35), suggests that MAPK signaling pathways are manipulated by CVB3 and that each pathway may have distinct cellular functions in relation to the viral life cycle.
The major function of MAPK signaling pathways is to regulate gene expression in response to extracellular stimuli (46). We previously found that CVB3 infection resulted in the induction of expression of c-Fos and c-Jun, which could hetero-dimerize to form activation protein 1 (AP-1) complexes (10, 32). AP-1 protein is a major transcription factor involved in cell proliferation, cellular and viral gene expression, and cell death and survival (43). Because the induction of AP-1 by stress is mediated mostly by the JNK1/2 and p38 MAPK pathways (43, 50), we investigated whether CVB3 infection resulted in activation of other components of AP-1, including ATF subfamilies (36). We examined the phosphorylation status of ATF-2, a common substrate for both JNK1/2 and p38 MAPK (18, 25, 28, 39), following CVB3 infection. We found that there was a marked increase in the phosphorylation of ATF-2 through the JNK1/2 pathway but not through the p38 MAPK pathway after CVB3 infection. The control of ATF-2 activation by the JNK1/2 pathway but not by the p38 MAPK pathway was surprising to us, because many other viruses primarily use the p38 MAPK pathway to activate ATF-2 (19, 38). The roles of increased JNK1/2 and ATF-2 phosphorylation during CVB3 infection remain to be elucidated. Recent studies from Kim et al. (23) suggest that CVB3 infection up-regulates cysteine-rich protein gene (cyr61) expression to mediate cell death via the JNK1/2 pathway; however, our results showed that inhibition of the JNK1/2 pathway did not prevent CVB3-induced caspase-3 activation and subsequent cell death. We speculate that activation of JNK/ATF-2 may be involved in the initiation of host antiviral mechanisms (40).
Specific inhibitors to JNK1/2 and p38 pathways allow us to dissect the individual roles of these pathways in viral replication. We found that inhibition of p38 MAPK but not of JNK1/2 was associated with reduced viral progeny release, improved cell viability, and decreased cell apoptosis in CVB3-infected cells. However, viral protein synthesis was unlikely to be affected by these inhibitors.
Previous studies have shown that virus infection often leads to p38 MAPK activation, although cellular functions of p38 MAPK vary and appear to be virus specific. Hirasawa et al. (19) reported that p38 MAPK was activated during EMCV replication and that cellular activity of p38 MAPK was required for efficient translation and transcription of EMCV viral RNA. Inhibition of p38 MAPK was associated with reduced replication of EMCV and Mengovirus (19), both cardiospecific picornaviruses. However, such inhibition has no effect on replication of human rhinovirus (17) or coxsackievirus B4 (19), the latter being an enterovirus closely related to CVB3. Activation of p38 MAPK was also observed in replication of human immunodeficiency virus (11, 42), herpes simplex virus (31), murine coronavirus (3), Sindbis virus (34), and varicella-zoster virus (38), but its cellular effects varied. The marked reduction of viral progeny release, along with the apparent lack of reduction in viral protein synthesis, upon inhibition of p38 MAPK suggests that p38 MAPK is not involved in suppressing internal ribosome entry site-dependent translation in the CVB3 life cycle, contrary to its role in the EMCV life cycle (19). While the exact cellular mechanisms leading to activation of p38 MAPK remain elusive, our data suggest that its activation is unlikely to be triggered by CVB3-induced oxidative stress. It has been reported that viral progeny release requires apoptosis of infected cells (6). In the current study, we have demonstrated that inhibition of p38 MAPK results in reduced apoptosis and increased cell viability, which likely contributes to the decreased viral progeny release.
In summary, we have demonstrated that CVB3 infection results in independent activation of the JNK1/2 and p38 MAPK pathways. Activation of p38 MAPK but not of JNK1/2 plays an important role in regulating CVB3-induced apoptosis, which is required for efficient viral progeny release. Taken together, our data strongly suggest that SAPK pathways play important roles in the pathogenesis of CVB3 infection.
Acknowledgments
This work was supported by grants from the Heart and Stroke Foundation of New Brunswick (to B.M.M.) and the Canadian Institutes of Health Research (CIHR) (to B.M.M. and H.L.). X.S. is a recipient of the CIHR IMPACT Post-Doctoral Fellowship, CIHR Michael Smith Fellowship, and the Heart and Stroke Foundation of Canada (HSFC) Research Fellowship. H.L. is a New Investigator of the CIHR/St. Paul's Hospital Foundation Award and a Scholar of the Michael Smith Foundation for Health Research (MSFHR). J.Y. is a recipient of a Doctoral Traineeship from the HSFC, CIHR, and the MSFHR. G.G. is a recipient of a Doctoral Traineeship from the HSFC and the MSFHR. C.C. is supported by a Doctoral Traineeship from the CIHR, and M.E. is supported by the Heart and Stroke Foundation of British Columbia and Yukon and the MSFHR.
REFERENCES
- 1.Anderson, D. R., J. E. Wilson, C. M. Carthy, D. Yang, R. Kandolf, and B. M. McManus. 1996. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J. Virol. 70:4632-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayach, B., K. Fuse, T. Martino, and P. Liu. 2003. Dissecting mechanisms of innate and acquired immunity in myocarditis. Curr. Opin. Cardiol. 18:175-181. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carthy, C. M., D. J. Granville, K. A. Watson, D. R. Anderson, J. E. Wilson, D. Yang, D. W. Hunt, and B. M. McManus. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carthy, C. M., B. Yanagawa, H. Luo, D. J. Granville, D. Yang, P. Cheung, C. Cheung, M. Esfandiarei, C. M. Rudin, C. B. Thompson, D. W. Hunt, and B. M. McManus. 2003. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology 313:147-157. [DOI] [PubMed] [Google Scholar]
- 7.Carthy, C. M., D. Yang, D. R. Anderson, J. E. Wilson, and B. M. McManus. 1997. Myocarditis as systemic disease: new perspectives on pathogenesis. Clin. Exp. Pharmacol. Physiol. 24:997-1003. [DOI] [PubMed] [Google Scholar]
- 8.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y. R., C. F. Meyer, and T. H. Tan. 1996. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. J. Biol. Chem. 271:631-634. [DOI] [PubMed] [Google Scholar]
- 10.Chiu, R., W. J. Boyle, J. Meek, T. Smeal, T. Hunter, and M. Karin. 1988. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 54:541-552. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, P. S., H. Schmidtmayerova, J. Dennis, L. Dubrovsky, B. Sherry, H. Wang, M. Bukrinsky, and K. J. Tracey. 1997. The critical role of p38 MAP kinase in T cell HIV-1 replication. Mol. Med. 3:339-346. [PMC free article] [PubMed] [Google Scholar]
- 12.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, K. A., N. M. Chapman, and S. D. Carson. 2003. Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res. 92:179-186. [DOI] [PubMed] [Google Scholar]
- 14.de Jong, A. S., E. Wessels, H. B. Dijkman, J. M. Galama, W. J. Melchers, P. H. Willems, and F. J. van Kuppeveld. 2003. Determinants for membrane association and permeabilization of the coxsackievirus 2B protein and the identification of the Golgi complex as the target organelle. J. Biol. Chem. 278:1012-1021. [DOI] [PubMed] [Google Scholar]
- 15.Esfandiarei, M., H. Luo, B. Yanagawa, A. Suarez, D. Dabiri, J. Zhang, and B. M. McManus. 2004. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J. Virol. 78:4289-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauntt, C., and S. Huber. 2003. Coxsackievirus experimental heart diseases. Front. Biosci. 8:e23-e35. [DOI] [PubMed] [Google Scholar]
- 17.Griego, S. D., C. B. Weston, J. L. Adams, R. Tal-Singer, and S. B. Dillon. 2000. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J. Immunol. 165:5211-5220. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, S., D. Campbell, B. Derijard, and R. J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389-393. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa, K., A. Kim, H. S. Han, J. Han, H. S. Jun, and J. W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huot, J., F. Houle, F. Marceau, and J. Landry. 1997. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 80:383-392. [DOI] [PubMed] [Google Scholar]
- 21.Kandolf, R., and P. H. Hofschneider. 1985. Molecular cloning of the genome of a cardiotropic coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc. Natl. Acad. Sci. USA 82:4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin, M. 1998. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann. N. Y. Acad. Sci. 851:139-146. [DOI] [PubMed] [Google Scholar]
- 23.Kim, S. M., J. H. Park, S. K. Chung, J. Y. Kim, H. Y. Hwang, K. C. Chung, I. Jo, S. I. Park, and J. H. Nam. 2004. Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. J. Virol. 78:13479-13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landry, J., H. Lambert, M. Zhou, J. N. Lavoie, E. Hickey, L. A. Weber, and C. W. Anderson. 1992. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 267:794-803. [PubMed] [Google Scholar]
- 25.Liu, F., and M. R. Green. 1990. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell 61:1217-1224. [DOI] [PubMed] [Google Scholar]
- 26.Liu, P., K. Aitken, Y. Y. Kong, M. A. Opavsky, T. Martino, F. Dawood, W. H. Wen, I. Kozieradzki, K. Bachmaier, D. Straus, T. W. Mak, and J. M. Penninger. 2000. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 6:429-434. [DOI] [PubMed] [Google Scholar]
- 27.Liu, P. P., and M. A. Opavsky. 2000. Viral myocarditis: receptors that bridge the cardiovascular with the immune system? Circ. Res. 86:253-254. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone, C., G. Patel, and N. Jones. 1995. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 14:1785-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin, U., M. Nestler, T. Munder, R. Zell, H. H. Sigusch, and A. Henke. 2004. Characterization of coxsackievirus B3-caused apoptosis under in vitro conditions. Med. Microbiol. Immunol. 193:133-139. [DOI] [PubMed] [Google Scholar]
- 31.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McManus, B. M., B. Yanagawa, N. Rezai, H. Luo, L. Taylor, M. Zhang, J. Yuan, J. Buckley, T. Triche, G. Schreiner, and D. Yang. 2002. Genetic determinants of coxsackievirus B3 pathogenesis. Ann. N. Y. Acad. Sci. 975:169-179. [DOI] [PubMed] [Google Scholar]
- 33.Mendelson, K. G., L. R. Contois, S. G. Tevosian, R. J. Davis, and K. E. Paulson. 1996. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc. Natl. Acad. Sci. USA 93:12908-12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakatsue, T., I. Katoh, S. Nakamura, Y. Takahashi, Y. Ikawa, and Y. Yoshinaka. 1998. Acute infection of Sindbis virus induces phosphorylation and intracellular translocation of small heat shock protein HSP27 and activation of p38 MAP kinase signaling pathway. Biochem. Biophys. Res. Commun. 253:59-64. [DOI] [PubMed] [Google Scholar]
- 35.Opavsky, M. A., T. Martino, M. Rabinovitch, J. Penninger, C. Richardson, M. Petric, C. Trinidad, L. Butcher, J. Chan, and P. P. Liu. 2002. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Investig. 109:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persengiev, S. P., and M. R. Green. 2003. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis 8:225-228. [DOI] [PubMed] [Google Scholar]
- 37.Qadri, I., M. Iwahashi, J. M. Capasso, M. W. Hopken, S. Flores, J. Schaack, and F. R. Simon. 2004. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem. J. 378:919-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahaus, M., N. Desloges, and M. H. Wolff. 2004. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 85:3529-3540. [DOI] [PubMed] [Google Scholar]
- 39.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reimold, A. M., J. Kim, R. Finberg, and L. H. Glimcher. 2001. Decreased immediate inflammatory gene induction in activating transcription factor-2 mutant mice. Int. Immunol. 13:241-248. [DOI] [PubMed] [Google Scholar]
- 41.Saraste, A., A. Arola, T. Vuorinen, V. Kyto, M. Kallajoki, K. Pulkki, L. M. Voipio-Pulkki, and T. Hyypia. 2003. Cardiomyocyte apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc. Pathol. 12:255-262. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro, L., K. A. Heidenreich, M. K. Meintzer, and C. A. Dinarello. 1998. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc. Natl. Acad. Sci. USA 95:7422-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131-E6. [DOI] [PubMed] [Google Scholar]
- 44.Si, X., B. M. McManus, J. Zhang, J. Yuan, C. Cheung, M. Esfandiarei, A. Suarez, A. Morgan, and H. Luo. 2005. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J. Virol. 79:8014-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokoe, D., K. Engel, D. G. Campbell, P. Cohen, and M. Gaestel. 1992. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 313:307-313. [DOI] [PubMed] [Google Scholar]
- 46.Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205-215. [DOI] [PubMed] [Google Scholar]
- 47.van Kuppeveld, F. J., J. G. Hoenderop, R. L. Smeets, P. H. Willems, H. B. Dijkman, J. M. Galama, and W. J. Melchers. 1997. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 16:3519-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Kuppeveld, F. J., W. J. Melchers, K. Kirkegaard, and J. R. Doedens. 1997. Structure-function analysis of coxsackie B3 virus protein 2B. Virology 227:111-118. [DOI] [PubMed] [Google Scholar]
- 49.Wada, T., and J. M. Penninger. 2004. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838-2849. [DOI] [PubMed] [Google Scholar]
- 50.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 51.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 52.Yuan, J. P., W. Zhao, H. T. Wang, K. Y. Wu, T. Li, X. K. Guo, and S. Q. Tong. 2003. Coxsackievirus B3-induced apoptosis and caspase-3. Cell Res. 13:203-209. [DOI] [PubMed] [Google Scholar]