Abstract
The emerging role of CD40, a tumor necrosis factor (TNF) receptor family member, in immune regulation, disease pathogenesis, and cancer therapy necessitates the analysis of CD40 signal transduction in a wide range of tissue types. In this study we present evidence that the CD40-interacting proteins TRAF2 and TRAF6 play an important physiological role in CD40 signaling in nonhemopoietic cells. Using mutational analysis of the CD40 cytoplasmic tail, we demonstrate that the specific binding of TRAF2 to CD40 is required for efficient signaling on the NF-κB, Jun N-terminal protein kinase (JNK), and p38 axis. In fibroblasts lacking TRAF2 or in carcinoma cells in which TRAF2 has been depleted by RNA interference, the CD40-mediated activation of NF-κB and JNK is significantly reduced, and the activation of p38 and Akt is severely impaired. Interestingly, whereas the TRAF6-interacting membrane-proximal domain of CD40 has a minor role in signal transduction, studies utilizing TRAF6 knockout fibroblasts and RNA interference in epithelial cells reveal that the CD40-induced activation of NF-κB, JNK, p38, and Akt requires the integrity of TRAF6. Furthermore, we provide evidence that TRAF6 regulates CD40 signal transduction not only through its direct binding to CD40 but also indirectly via its association with TRAF2. These observations provide novel insight into the mechanisms of CD40 signaling and the multiple roles played by TRAF6 in signal transduction.
The tumor necrosis factor (TNF) receptor superfamily and its ligands are increasingly recognized as key regulators of a plethora of fundamental biological processes, including apoptosis, immune regulation, and inflammation (reviewed in reference 41). CD40 is a member of the TNF receptor family, which has attracted much attention because of its pivotal role in the generation of adaptive and innate immune responses. Thus, the interaction of CD40 expressed on B lymphocytes with the CD40 ligand (CD40L/CD154), which is found predominantly on activated T cells, is critical in inducing B-cell immunity by driving the formation of germinal centers and the proliferation and differentiation of resting B lymphocytes into immunoglobulin-producing plasma cells (reviewed in references 47 and 57). CD40 is also expressed on other lymphoid cell types, such as macrophages and dendritic cells where its activation contributes to antigen presentation and the production of cytokines that influence T-cell priming and the innate immune response to a variety of extracellular and intracellular pathogens (57).
These pleiotropic functions of CD40 have sparked an immense interest in the mechanisms of CD40 signal transduction. Studies in normal B lymphocytes and B-cell lines have shown that CD40 ligation causes the activation of the Jun N-terminal protein kinase (JNK), p38 mitogen-activated protein kinases (MAPK), the phosphatidylinositol 3 (PI3)-kinase/Akt pathway and the transcription factor NF-κB (3, 5, 20, 39). The cytoplasmic tail of CD40 lacks intrinsic catalytic activity and signals largely through its ability to recruit TNF receptor-associated factors (TRAFs), adapter proteins that bridge receptors of the TNF family to downstream signaling pathways. Of the six known mammalian TRAFs, TRAF2 and TRAF3 directly bind to a membrane-distal CD40 cytoplasmic domain (32, 37), whereas TRAF6 weakly interacts with a membrane-proximal site (56) (Fig. 1A). TRAF5 has also been reported to directly interact with the TRAF2/TRAF3 binding site of CD40 (56), but other studies indicate that this interaction is indirect and occurs via TRAF3 (38). Irrespective of the nature of the CD40/TRAF5 association, studies utilizing B lymphocytes from TRAF5−/− mice demonstrate normal JNK and NF-κB activation in response to CD40 stimulation and, therefore, exclude a major physiological role for TRAF5 in CD40 signal transduction (43).
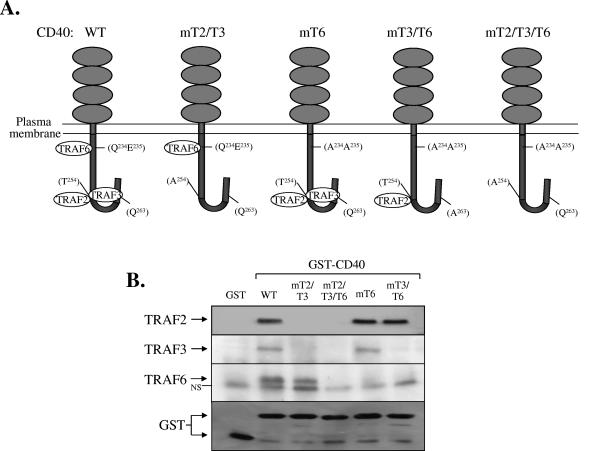
FIG. 1.
Point mutations in the cytoplasmic tail of CD40 selectively disrupt direct associations with TRAF proteins. (A) Schematic representation of WT and mutated CD40 sequences used in this study (see text for details). (B) Direct interactions of WT and mutated CD40 sequences with TRAF2, TRAF3, or TRAF6 were determined by GST pull-down assays using lysates isolated from HeLa cervical carcinoma cells, as described in Materials and Methods. NS, nonspecific.
Although overexpression of TRAF2 or TRAF6 activates the NF-κB and MAPK pathways (4, 44, 51), the precise role of these adapter proteins in CD40-induced signaling is not well defined. Mutational analysis of the human and mouse CD40 cytoplasmic tails and overexpression studies utilizing dominant-negative TRAF mutants have previously yielded conflicting results on the relative contribution of these TRAF molecules to CD40 signal transduction (2, 34, 38, 56). These discrepancies could be at least partly attributed to the type of agonistic stimulation used (CD40 antibody versus soluble ligand or overexpression-induced CD40 oligomerization) and the relative levels of TRAF protein expression in different cell lines. More recently, the physiological role of TRAF2 in CD40-mediated NF-κB activation has been addressed in murine TRAF2−/− B cells. These studies showed that whereas TRAF2 significantly contributes to CD40-induced NF-κB activation in normal mouse B lymphocytes (27, 45), it plays no role in NF-κB signaling in transformed B cells (31). Interestingly, a severe defect in CD40-mediated NF-κB binding activity and cytokine synthesis has been reported in TRAF6-deficient mouse B lymphocytes (40) and dendritic cells (36), respectively. The physiological contribution of TRAF2 and TRAF6 to other CD40L-induced signals, such as JNK, p38, and Akt is currently unknown.
Although these studies have largely focused on B cells, CD40 is also expressed in a wide range of other cell types, including fibroblasts and neuronal, endothelial, and epithelial cells, raising the possibility that the CD40 pathway may have a broader contribution to human physiology and disease pathogenesis. Indeed, the expression of CD40 on rheumatoid synovial fibroblasts has been proposed to facilitate inflammation via interaction with T lymphocytes and mast cells, which display the CD40 ligand (8, 50). Moreover, CD40 expressed in human lung fibroblasts transduces signals that regulate the synthesis of the proinflammatory prostaglandin E2, and disruption of the CD40/CD40L pathway decreases lung inflammation and fibrosis (1, 63). CD40 is also detected in normal human but not mouse epithelial cells (14, 29, 42, 61), where its activation results in suppression of proliferation and induction of differentiation (17, 48). The overexpression of CD40 in various types of carcinoma has also attracted much attention because of the emerging potential of CD40L-based cancer therapies (reviewed in references 21 and 54). Indeed, CD40 ligation in carcinoma cells has been shown to confer direct antiproliferative and proapoptotic effects in vitro and in vivo (16, 17, 24, 28-30) and to enhance their in vitro recognition and killing by antigen-specific CD8+ cytotoxic T lymphocytes (29). This widespread expression of functional CD40 therefore necessitates the analysis of CD40 signaling and of the contribution of TRAF proteins to CD40 signal transduction in a variety of different cell types.
In this study, we present the first evidence that TRAF2 and TRAF6 play an important physiological role in CD40 signaling in epithelial cells and fibroblasts. Our data disclose a mechanism of CD40 signal transduction that requires not only the direct binding of TRAF6 to CD40 but also its function downstream of the CD40/TRAF2 signaling complex. These observations provide novel insight into the mechanisms of CD40 signaling and the multiple roles played by TRAF6 in signal transduction.
MATERIALS AND METHODS
Constructs.
Point mutations in the cytoplasmic tail of CD40 were generated from the pCDNA3neo/CD40 expression vector (18) using a Stratagene Quick Change site-directed mutagenesis kit according to the manufacturer's instructions. The following primers were utilized for the site-directed mutagenesis reactions with the desired mutations underlined: 5′-GCTCCAGTGCAGGAGGCTTTACATGGATGCC-3′ and its complementary oligonucleotide to generate a T254→A mutation; 5′-CAAGCAGGAACCCGCGGCGATCAATTTTCCCG-3′ and its complementary oligonucleotide to generate Q234E235→AA; and 5′-GCCAACCGGTCACCGCGGAGGATGGCAAAGAG-3′ and its complementary oligonucleotide to generate Q263→A. Double mutants were generated on the backbone of single mutations.
Generation of GST-CD40 fusion proteins and GST-CD40 pull-downs.
Glutathione transferase (GST) fusion proteins of the cytoplasmic tail of wild-type and mutated CD40 were generated by PCR amplification using pCDNA3/CD40 and mutated CD40 receptors as templates and the following primers: 5′-GAATTCAAAAAGGTGGCCAAGAAGCCAACC-3′ (CD40CT-FORW; underlined sequence denotes an artificial EcoRI site) and 5′-TGGGTGGCGGCCGCCTCACT-3′ (CD40CT-REV). The PCR products were digested with EcoRI/NotI and cloned into the respective sites of pGEX-5X-1 (Pharmacia). Escherichia coli BL21 cells (Invitrogen) were transformed with pGEX-5X-1/CD40 fusions, grown to an optical density of 0.4, and induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside for 4 h. Bacterial cultures were lysed by pulse sonication in buffer A (20 mM Tris, pH 7.6, 0.5% Triton X-100, 250 mM NaCl, 3 mM EGTA, 3 mM EDTA, 1 mM Na3VO4, 50 mM NaF, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, 1 μg/ml aprotinin). Soluble GST-CD40 proteins were purified by incubating overnight at 4°C with GST Sephrose 4B beads (Amersham) and washed three times with buffer A, and the resultant Sepharose beads/GST-CD40 complex was resuspened in a volume of buffer A to obtain a 1:4 slurry. For GST-CD40 pull-downs, unstimulated HeLa cell cultures were lysed with lysis buffer B (20 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM Na3VO4, 50 mM NaF, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, 1 μg/ml aprotinin) and 2 mg of protein incubated with 20 μl of Sepharose/GST-CD40 beads for 2 h at 4°C. The beads were washed three times with buffer B and boiled in the presence of sodium dodecyl sulfate (SDS) gel loading buffer, and the proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) prior to immunoblotting and detection of GST-CD40/TRAF associations.
Cell culture, retrovirus infections, flow cytometry, reporter assays, and ELISA.
The cervical carcinoma cell line HeLa and the bladder carcinoma cell line EJ were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HeLa-CD40 and HeLa-CD40 mutants were generated as previously described (16). Fibroblasts were generated from mice maintained under pathogen-free conditions and in accordance with institutional and University of Birmingham guidelines. Newborn TRAF2−/− mice are smaller in size than their wild-type equivalents, become progressively runted, and die within 2 weeks of birth (60). We therefore established fibroblasts from tongues of 1-week-old TRAF2+/+ and TRAF2−/− mice. These primary cells were then immortalized by simian virus 40 large T antigen to increase the transfection efficiency and allow the generation of stable lines transfected with human CD40 in pcDNA3hygro vector. Selection was performed in 100 to 300 mg/ml hygromycin (Roche); single colonies were expanded and analyzed for CD40 expression by immunoblot. TRAF6+/+ and TRAF6−/− fibroblasts were generated in a similar manner. For the experiments involving infections with TRAF retroviruses, TRAF2 or TRAF6 was first cloned under the control of the stem cell virus promoter in pMSCV (where MSCV is mouse stem cell virus) vector (kindly provided by P. N. Tsichlis, Tufts NEMC, Boston, MA). TRAF2−/− CD40 and TRAF6−/− CD40 fibroblasts were infected with TRAF2- and TRAF6-expressing retroviruses, respectively, by exposure to supernatant from transiently transfected Phoenix 293 cells in two successive 12-h incubations in the presence of polybrene, followed by selection with puromycin for 2 weeks and subsequent expansion of resistant clones. Cells were treated with recombinant soluble CD40L purchased from Caltag MedSystems (Austria) or with human or murine interleukin-1 (IL-1) purchased from R&D Systems. Flow cytometry using the anti-CD40 monoclonal antibody (MAb) G28.5 and reporter assays were performed as previously described (19, 29).
RNA interference.
For delivery of small interfering RNAs (siRNAs), 1.2 × 105 HeLa-CD40mT6 cells or 8 × 104 EJ bladder carcinoma cells were plated into each well of a 24-well plate (Costar), and the next day siRNA duplexes were transfected using the siIMPORTER transfection reagent (Upstate Biotechnology). For each well transfected, 3.5 μl of siIMPORTER was gently mixed with 25 μl of Opti-MEM (Gibco). In a separate Eppendorff tube, 15 μl of Opti-MEM, 10 μl of dilution buffer, and the appropriate siRNA duplex were mixed and then combined with siIMPORTER/Opti-MEM for 5 min. Transfection cocktails were then added by drops to cells and supplemented with Opti-MEM to give a final volume of 250 μl. All siRNA duplexes were added to obtain a final concentration of 200 nM per 250 μl of transfection volume. TRAF2 was targeted by cotransfecting two TRAF2 siRNA duplexes at a final concentration of 100 nM each (AUACGAGAGCUGCCACGAAdTdT and AGAGGCCAGUCAACGACAUdTdT and their antisense sequences), while TRAF6 was targeted with the siRNA duplex (CUGUGCUGCAUCAAUGGCAdTdT) (64). All siRNA duplexes were synthesized by MWG, Germany. As a control, an siRNA targeting the luciferase gene (CUUACGCUGAGUACUUCGCAdTdT) was used (Upstate, Lake Placid, NJ). Following a 6-h incubation with TRAF2, TRAF6, or control siRNA duplexes, an equal volume of medium supplemented with 20% fetal calf serum was added. Cells were then left to recover for 10 h and were replated in 24-well dishes. The next day, the cells were subjected to a second round of transfection with siRNAs to increase the siRNA-mediated gene suppression. Forty-eight hours later, cultures were stimulated with CD40L before lysis.
Antibodies and immunoblotting.
Phospho-specific antibodies were diluted in 5% bovine serum albumin in Tris-buffered saline containing 0.1% (vol/vol) Tween 20 (TBS-T) while all other antibodies were diluted in 5% milk in TBS-T. Phospho-p38 MAPK (Thr180/Tyr182), phospho-Akt (Ser473), phospho-JNK (Thr183/Tyr185), and the corresponding antibodies which recognize “total” forms, i.e., both phosphorylated and nonphosphorylated, were purchased from Cell Signaling Technology and used at a dilution of 1:1,000. The IκBα (C21), TRAF2 (C-20), TRAF3 (H122), TRAF6 (D10 and H274), JNK1 (C17), p65 (C20), and p50 (H199) antibodies were purchased from Santa-Cruz Biotechnology, and β-actin was from Sigma. Anti-rabbit immunoglobulin G-horseradish peroxidase (1:5,000) was purchased from Pierce, and anti-mouse immunoglobulin G-horseradish peroxidase (1:1,000) was from Sigma. For immunoblotting, 15 to 30 μg of protein was separated by SDS-PAGE, transferred onto polyvinylidene difluoride membrane for phospho-specific immunoblotting (0.45 μM; Millipore) or nitrocellulose membrane (Pall Gelman Laboratory, Portsmouth, United Kingdom) and blocked for 60 min at room temperature with 5% nonfat milk dissolved in TBS-T. Following three washes with TBS-T, membranes were incubated overnight at 4°C with primary antibody and for 1 h at room temperature with the appropriate secondary antibody, followed by enhanced chemiluminescence (ECL; Amersham).
Immunoprecipitations.
CD40-expressing HeLa cells were seeded at 5 × 106 cells per 10-cm plate and stimulated with 0.1 μg/ml recombinant CD40L. Following three washes with ice-cold phosphate-buffered saline, cells were lysed in situ with 1 ml of lysis buffer C (20 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5 mM CaCl2, 1% Brij 98, 1 mM Na3VO4, 50 mM NaF, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin A). TRAF2 was immunoprecipitated by incubating 3 mg of protein with 2 μg of anti-TRAF2 C20 antibody (Santa Cruz Biotechnology) overnight at 4°C. Complexes were then captured by incubation with 25 μl of protein A beads for 2 h and then washed three times with lysis buffer C using slow-spin centrifugation (4,000 rpm for 2 min) to pellet the beads. Beads were drained with a Hamilton glass syringe and resuspended with 40 μl of SDS gel sample buffer. The samples were boiled, and 8 μl of the total immunoprecipitation was immunoblotted for TRAF2 using a monoclonal antibody (Alexis Corp, Nottingham, United Kingdom), while the remaining 30 μl was immunoblotted for TRAF6 using the MAb D-10 (Santa Cruz Biotechnology).
Preparation of nuclear extracts and EMSAs.
Cells were resuspended in 5 volumes of homogenization buffer (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]) supplemented with protease inhibitors (0.1 mM sodium orthovanadate, 10 mM sodium glycerophosphate, 2 μg/ml leupeptin, and 2 μg/ml aprotinin) and centrifuged at 1,200 rpm for 10 min. The pellet was resuspended in 3 volumes of ice-cold homogenization buffer containing 0.05% NP-40, and cells were homogenized with 15 to 20 strokes of a tight-fitting Dounce homogenizer on ice. Following centrifugation for 10 min at 1,200 rpm at 4°C, the cytoplasmic fraction was collected. The nuclei were washed once with homogenization buffer and then resuspended in buffer containing 40 mM HEPES-KOH, pH 7.9, 0.4 M KCl, 1 mM DTT, and 10% glycerol with protease inhibitors. NaCl was added at a final concentration of 300 mM; the suspension was mixed and incubated on ice for 30 min and then centrifuged at 24,000 rpm for 30 min. The supernatants were removed and snap frozen. Proteins were analyzed for the expression of NF-κB subunits by immunoblotting. The electrophoretic mobility shift assays (EMSAs) using the human immunodeficiency virus long terminal repeat (HIV-LTR) NF-κB probe, which strongly binds p65-containing NF-κB complexes, were performed as previously described (20).
JNK assays.
Cells were lysed with kinase lysis buffer (20 mM Tris, pH 7.6, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2.5% Triton X-100, 1 mM Na3VO4, 50 mM NaF, 10 mM β-glycerophosphate, 0.5 mM sodium pyrophosphate, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 0.1% β-mercaptoethanol) and JNK1 was immunoprecipitated by incubating 75 to 100 μg of cell lysate with 1 μg of JNK1 C-17 antibody (Santa Cruz Biotechnology) for 2 h. Complexes were then captured with 25 μl of a 1:1 slurry of protein G beads (Amersham) and washed twice in kinase lysis buffer and three times in assay dilution buffer (20 mM HEPES, pH 7.5, 20 mM β-glycerophosphate, 10 mM MgCl2, 1 mM DTT, 0.1 mM Na3VO4). Kinase reactions were initiated by resuspending beads in 40 μl of assay dilution buffer containing 1 μg of c-Jun fusion protein (Cell Signaling Technology), 5 μCi of [γ-32P]ATP and 25 μM “cold” ATP for 30 min at 30°C. Proteins were resolved by SDS-PAGE, and the lower part of the gel was dried and exposed to autoradiography while the upper section was immunoblotted to determine levels of immunoprecipitated JNK1.
RESULTS
Generation of point mutations in the cytoplasmic tail of CD40 which disrupt TRAF binding.
To address the impact of specific CD40-TRAF interactions on CD40L-induced signal transduction in nonhemopoietic cells, we generated a series of mutated (m) human CD40-expression constructs, which are illustrated in Fig. 1A. Construct CD40mT2/T3 contains a T254→A point mutation that has been shown to abolish the binding of both TRAF2 and TRAF3 to CD40 but does not affect interactions with TRAF6 (34, 56). Construct CD40mT6 carries a Q234E235→AA double mutation that only disrupts interactions with TRAF6 (56), and CD40mT2/T3/T6 carries a triple mutation of CD40 with Q234E235T254→AAA. To determine whether these CD40 mutants lack the expected direct associations with TRAF molecules, fusions of GST with the cytoplasmic tails of wild-type (WT) or mutated CD40 proteins were examined for their ability to interact with TRAFs in HeLa cell lysates, as previously described for other cell types (2, 34, 56). The results (Fig. 1B) confirmed that GST-CD40WT and GST-CD40mT6 directly interact with TRAF2 and TRAF3, whereas GST-CD40mT2/T3 does not. In contrast, both GST-CD40WT and GST-CD40mT2/T3, but not GST-CD40mT6, were found to bind TRAF6. GST-CD40mT2/T3/T6 did not interact with any of the TRAF molecules examined (Fig. 1B). As an additional control, GST alone also failed to bind TRAFs.
The T254→A point mutation abolishes both TRAF2 and TRAF3 binding. To facilitate the analysis of the relative impact of TRAF2 versus TRAF3 on CD40 signal transduction, we generated a novel mutated receptor, CD40mT3/T6 (Fig. 1A). This molecule was constructed on the basis of recent crystallographic data showing that Q263 is important for the interaction of CD40 with TRAF3 but not TRAF2 (46). A Q263→A mutation was therefore introduced on the backbone of CD40mT6 which does not bind TRAF6. A fusion of GST with the cytoplasmic tail of CD40mT3/T6 was examined for its ability to interact with endogenous TRAFs in HeLa cell lysates. This mutated receptor was found to maintain direct interactions only with TRAF2, whereas its ability to associate with TRAF3 was significantly compromised (Fig. 1B). Indeed, in three independent experiments the Q263→A mutation consistently reduced the CD40-TRAF3 interaction by more than 90%.
The binding of TRAF2 to the cytoplasmic tail of CD40 plays a major role in CD40 signal transduction in nonhemopoietic cells.
The WT and mutated CD40 expression vectors described in Fig. 1A were transfected in CD40-negative HeLa cells, and stable clones were obtained. Flow cytometric analysis was performed to select clones with similar levels of CD40 expression (Fig. 2A). These cells were stimulated with human trimeric soluble CD40L for various time intervals, and lysates were examined for the activation of p38 and JNK by immunoblotting using either antibodies that detect the phosphorylated, active form of these proteins or antibodies that detect p38 and JNK irrespective of phosphorylation status. The results demonstrated robust phosphorylation of p38 and JNK in CD40L-stimulated HeLa-CD40 cells which was unaffected by the Q234E235→AA mutation (CD40mT6) but was severely impaired in both CD40mT2/T3- and CD40mT2/T3/T6-expressing HeLa clones (Fig. 2B and C). Therefore, the association of TRAF2 and/or TRAF3, but not TRAF6, with the cytoplasmic tail of CD40 plays a major role in CD40L-induced JNK and p38 signaling. To determine whether the specific interaction of CD40 with TRAF2 or TRAF3 is responsible for these activities, we analyzed the effects of CD40L on HeLa cells expressing a mutated CD40 (CD40mT3/T6) that interacts only with TRAF2 but not TRAF3 or TRAF6. The results showed that the absence of TRAF3 binding to CD40 had only a small impact on CD40L-induced p38 and JNK signaling (Fig. 2B and C). We conclude that the CD40-transduced JNK and p38 signals largely depend on the CD40-TRAF2 interaction.
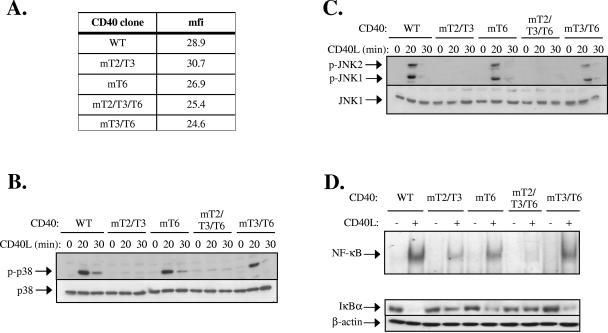
FIG. 2.
The binding of TRAF2 to the cytoplasmic tail of CD40 plays a major role in CD40 signal transduction in nonhemopoietic cells. (A) Representative flow cytometric assay showing the CD40 levels expressed in HeLa cell clones stably transfected with wild type and mutated CD40 sequences. mfi, mean fluorescence intensity. (B and C) Activation of p38 (B) and JNK (C) following CD40 activation in WT and mutated CD40-transfected HeLa cells. Cells were stimulated with 0.1 μg/ml recombinant soluble CD40L or left untreated, as indicated. Lysates were immunoblotted with antibodies specific for the phosphorylated or total p38 and JNK. At least three independent experiments were performed and gave similar results. In addition to results from the GST pull-down assays (Fig. 1B), the inability of CD40mT3/T6 to interact with TRAF3 was confirmed by immunoprecipitation assays in lysates from CD40L-stimulated HeLa-CD40mT3/T6 cells (data not shown). (D) Induction of NF-κB binding activity following CD40 activation in WT and mutated CD40-transfected HeLa cells. Cells were stimulated with 0.1 μg/ml CD40L or left untreated, as indicated. Nuclear proteins were isolated and examined for binding to a 32P-labeled oligonucleotide probe containing the HIV-LTR NF-κB binding site. Data were quantitated on a phosphorimager and expressed as the increase (n-fold) relative to unstimulated controls, which were given the arbitrary value of 1. The mean inducible increase (± standard deviation; n = 4 assays) in NF-κB binding was as follows: WT, 8.1 ± 1.6; CD40mT2/T3, 2.4 ± 0.3; CD40mT6, 5.7 ± 0.5; CD40mT2/T3/T6, 1.1 ± 0.05; and CD40mT3/T6, 5.3 ± 0.6. Supershift analysis using antibodies against the p65 and p50 NF-κB subunits confirmed that the DNA-bound proteins represent NF-κB (data not shown). Parallel cultures from a representative experiment were lysed and examined for IκBα or β-actin levels by immunoblotting.
CD40 ligation in lymphoid and epithelial cells induces the phosphorylation and subsequent degradation of IκBα, a cytoplasmic NF-κB inhibitory protein, allowing the release of the associated p65/p50 NF-κB subunits and their translocation to the nucleus. EMSA was used to determine the DNA binding activity of nuclear NF-κB before and 30 min after stimulation with CD40L. The results showed that the CD40L-induced NF-κB binding activity was partly reduced in HeLa-CD40mT6 and HeLa-CD40mT3/T6 cells, severely impaired in the CD40mT2/T3 HeLa cells, and essentially absent in CD40mT2/T3/T6 HeLa clones (Fig. 2D). The levels of IκBα were also assessed in parallel cultures. These experiments showed a dramatic reduction in IκBα expression in CD40L-stimulated HeLa-CD40 cells and a partial but significant reduction in CD40mT6-expressing cells (Fig. 2D). In contrast, only limited IκBα degradation was observed in CD40mT2/T3-transfected HeLa cells, consistent with the low level NF-κB binding activity seen in these cultures. Importantly, the absence of TRAF3 binding to CD40 had no significant impact on CD40L-induced IκBα degradation (Fig. 2D). Taken together, the preceding data suggest that the specific interaction of CD40 with TRAF2 is largely responsible for the CD40L-induced IκBα/NF-κB and MAPK signaling in nonhemopoietic cells.
RNAi-mediated knock-down of TRAF2 expression results in diminished CD40-induced NF-κB, JNK, and p38 activation in epithelial cells.
To examine the impact of the overall TRAF2 depletion on CD40 signal transduction, RNA interference (RNAi) was used to knock down TRAF2 expression in EJ bladder carcinoma cells which naturally express CD40 (17, 53). Transfection of these cells with TRAF2 siRNA significantly reduced the levels of TRAF2 compared to an unrelated siRNA targeting the luciferase gene. In contrast, expression of β-actin remained unaffected (Fig. 3A).
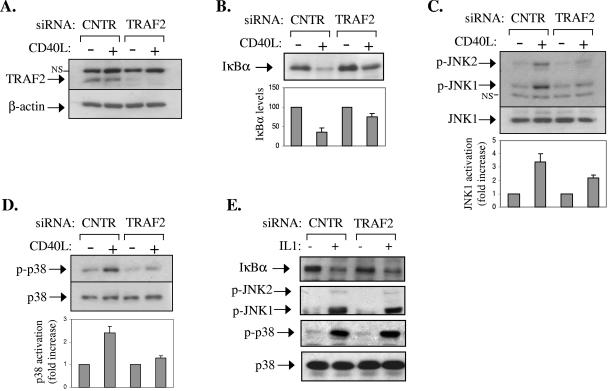
FIG. 3.
RNAi-mediated knock-down of TRAF2 expression results in diminished CD40-induced NF-κB, JNK, and p38 activation in epithelial cells. (A) EJ bladder carcinoma cells were transfected with siRNA targeting TRAF2 or the unrelated luciferase gene product. Lysates were analyzed for TRAF2 or β-actin levels by immunoblotting. (B to D) Parallel cultures were stimulated with CD40L or left untreated. Lysates were isolated and analyzed for the expression of IκBα (B) and of the phosphorylated and total JNK (C) and p38 (D) by immunoblotting. Data from three independent experiments were quantitated using the Scion Image processing and analysis software and are expressed as the increase (n-fold) relative to unstimulated controls. NS, nonspecific. (E) The RNAi-mediated knock-down of TRAF2 does not affect IL-1-induced signaling. Cells were transfected with TRAF2 or luciferase siRNA as described in panel A and stimulated with 15 ng/ml IL-1 for 20 min prior to evaluation of the endogenous IκBα levels and of JNK and p38 phosphorylation by immunoblotting.
Parallel transfected cultures were stimulated with CD40L for 10 min or left untreated. Lysates were then analyzed for IκBα degradation or JNK and p38 phosphorylation by immunoblotting. The results showed that TRAF2 protein knock-down significantly impairs the ability of CD40L to activate these signaling pathways (Fig. 3B to D). To exclude the possibility that the described RNAi findings are the result of unintended, nonspecific effects of the siRNA treatment, control experiments were performed. First, NF-κB, JNK, and p38 activities were measured in TRAF2 siRNA-transfected EJ cultures stimulated with IL-1, which operates in a TRAF2-independent manner. The results of this analysis showed normal IκBα degradation as well as JNK and p38 phosphorylation in these cells (Fig. 3E). Second, EJ cells were transfected with siRNA targeting an unrelated but expressed protein, namely Akt, and lysates were assessed for IκBα degradation before and after stimulation with CD40L. In agreement with our previous report on HeLa/CD40 cells (10), knock-down of Akt did not affect the CD40-mediated degradation of IκBα in EJ cell cultures (data not shown). On the basis of the preceding data, we conclude that TRAF2 plays an important physiological role in CD40 signal transduction in epithelial cells.
TRAF2 deficiency results in impaired CD40 signaling in fibroblasts.
To confirm that the effects of TRAF2 on CD40 signaling described in Fig. 3 are not restricted to epithelial cells, we examine the impact of the overall TRAF2 deficiency on CD40 signal transduction in fibroblasts. To this end, fibroblast cell lines from TRAF2−/− and TRAF2+/+ mice were established as described in Materials and Methods and stably transfected with a CD40 expression vector. Positive clones expressing similar levels of CD40 (Fig. 4A) were then selected for subsequent analysis. The absence of TRAF2 in the CD40-transfected knockout fibroblast clone was verified by immunoblotting (Fig. 4A).
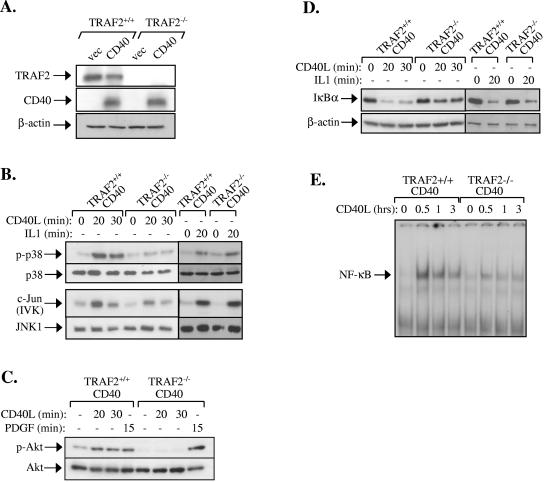
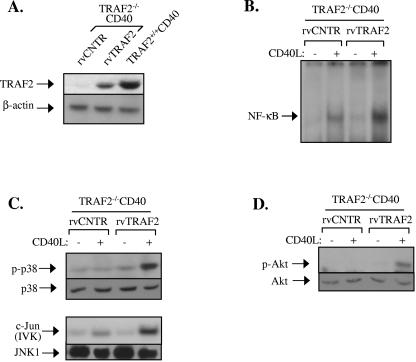
FIG. 4.
TRAF2 deficiency results in impaired CD40 signaling in fibroblasts. (A) Expression of TRAF2, CD40, and actin loading control in CD40-transfected immortalized TRAF2+/+ and TRAF2−/− fibroblasts, as determined by immunoblot analysis of total cell lysates. (B) Phosphorylation of p38 and activation of JNK are significantly reduced in CD40L-stimulated but not IL-1-treated TRAF2−/− CD40 fibroblasts. TRAF2+/+ CD40 and TRAF2−/− CD40 fibroblasts were stimulated with CD40L for 20 or 30 min or with murine IL-1 for 20 min or left untreated. Lysates were immunoblotted for the phosphorylated or total p38 or immunoprecipitated with an anti-JNK1 antibody. The anti-JNK1 immunoprecipitates were examined for activity toward c-Jun as a substrate or immunoblotted with a rabbit polyclonal JNK1 antibody. Data are representative of at least four independent experiments for the CD40L and two experiments for the IL-1 treatment. IVK, in vitro kinase assay. (C) Phosphorylation of Akt is impaired in CD40L-stimulated but not PDGF-treated TRAF2−/− CD40 fibroblasts. TRAF2+/+ CD40 and TRAF2−/− CD40 fibroblasts were treated with 0.5 μg/ml CD40L for 20 or 30 min, with 50 ng/ml PDGF, or left untreated as indicated. Lysates were then immunoblotted for the phosphorylated or total Akt. (D) IκBα degradation is diminished in CD40L-stimulated TRAF2−/− CD40 fibroblasts. TRAF2+/+ CD40 and TRAF2−/− CD40 fibroblasts were treated with CD40L or IL-1 or left untreated, as indicated. Lysates were examined for the expression levels of IκBα or, as a control, β-actin. (E) NF-κB binding activity is significantly reduced in CD40L-stimulated TRAF2−/− CD40 fibroblasts. Nuclear proteins were isolated at various time intervals following stimulation with CD40L and examined for binding to a 32P-labeled oligonucleotide probe containing the HIV-LTR NF-κB binding site. At least four independent experiments were performed and gave similar results. Supershift analysis using antibodies against the p65 and p50 NF-κB subunits confirmed that the DNA-bound proteins at 30 min stimulation represent NF-κB (data not shown).
Western blots of cell lysates harvested at sequential time points following CD40 stimulation were probed with antibodies against the phosphorylated, active p38 and its total (phosphorylated and nonphosphorylated) equivalent. Immunoblots were also probed for phosphorylated and total Akt, an established component of the PI3 kinase pathway (23). Finally, JNK activation was determined by in vitro kinase assays using GST-c-Jun as a substrate. The results showed that CD40L efficiently activates the JNK and p38 MAPKs and Akt in CD40-transfected TRAF2+/+ fibroblasts (Fig. 4B and C). However, the CD40-mediated activation of p38 and JNK was significantly reduced, and the phosphorylation of Akt was severely impaired in fibroblast clones deficient of TRAF2 (Fig. 4B and C). The observed effects were specific because the stimulation of TRAF2−/− CD40 fibroblasts with the TRAF2-independent stimuli IL-1 and platelet-derived growth factor (PDGF) resulted in normal JNK and p38 activation (Fig. 4B) and Akt phosphorylation (Fig. 4C), respectively. The CD40L- but not IL-1-mediated degradation of IκBα was also found to be impaired in TRAF2−/− CD40 cells (Fig. 4D). EMSA was then used to determine the DNA binding activity of nuclear NF-κB before and 30, 60, or 180 min after stimulation with CD40L. CD40 ligation was found to induce robust NF-κB activation in TRAF2+/+ CD40 cells, and this activity was significantly inhibited, but not abolished, in fibroblast clones deficient in TRAF2 (Fig. 4E).
Reconstitution experiments were then performed to confirm that the reduction in CD40-mediated signal transduction observed in TRAF2−/− CD40 fibroblasts was indeed TRAF2 dependent. In these experiments, TRAF2−/− CD40 cells were infected with a TRAF2-expressing retrovirus and either examined for expression of TRAF2 (Fig. 5A) or stimulated with CD40L and examined for the activation status of NF-κB, MAPK, and Akt (Fig. 5B to D). The results showed that exogenous TRAF2 restored the CD40L-induced signal transduction in TRAF2−/− CD40 cells.
FIG. 5.
Expression of TRAF2 restores the CD40 signaling deficiency in TRAF2−/− CD40 fibroblasts. Cells were infected with TRAF2 or control (CNTR) retrovirus (rv) in two successive 12-h incubations, as described in Materials and Methods. At 36 h postinfection, cells were reseeded and selected for puromycin resistance during a 2-week period. Antibiotic-resistant clones were expanded and either examined for TRAF2 expression (A), stimulated with CD40L for 0 or 30 min and analyzed for NF-κB binding activity (B), or stimulated with CD40L for 0 or 20 min and examined for the activation status of JNK or p38 MAPK (C) or Akt (D). IVK, in vitro kinase assay.
Collectively, the preceding data, coupled with the CD40 mutagenesis experiments shown in Fig. 2, demonstrate a prominent but not exclusive role for TRAF2 in CD40-mediated induction of NF-κB and MAPK signaling in nonhemopoietic cells.
CD40 critically depends on TRAF6 for signal transduction.
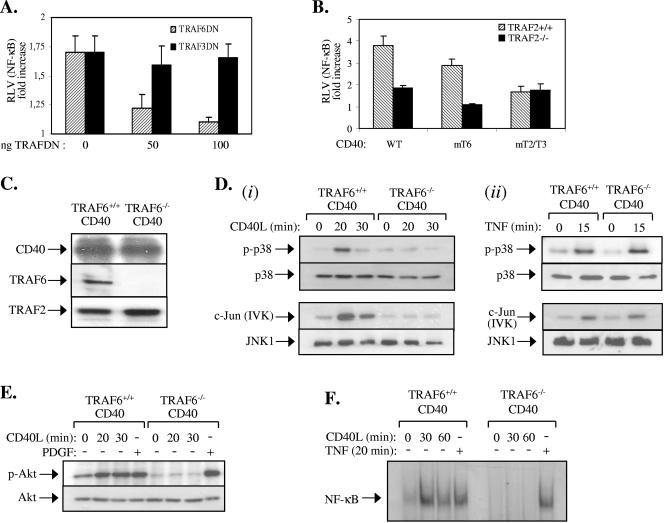
The residual NF-κB signaling in CD40L-stimulated TRAF2−/− CD40 fibroblasts (Fig. 4E) could be attributed to the binding of TRAF3 and/or TRAF6 to CD40. This issue was addressed by performing NF-κB reporter assays in TRAF2−/− CD40 cells following their transfection with low amounts of dominant-negative TRAF3 or TRAF6 mutants. The results showed that, whereas dominant-negative TRAF6 dramatically reduced the residual NF-κB transcriptional activity in CD40L-stimulated TRAF2−/− CD40 cells, dominant-negative TRAF3 had no effect (Fig. 6A). Moreover, we did not observe significant differences in the TRAF3 levels or the CD40-TRAF3 interaction between TRAF2−/− CD40 and TRAF2+/+ CD40 cells (see Fig. S1 in the supplemental material). To confirm the importance of the direct TRAF6 binding to CD40 in this effect, TRAF2−/− and TRAF2+/+ cells were transiently transfected with wild-type CD40, CD40mT6, or CD40mT2/T3 in the presence of an NF-κB-responsive luciferase reporter plasmid. Cells were stimulated with CD40L, and lysates were examined for NF-κB transcriptional activity. The results from multiple experiments showed that CD40mT6 fails to transduce NF-κB activation signals in TRAF2−/− cells (Fig. 6B). However, the ability of a CD40 mutant that only binds TRAF6 (CD40mT2/T3) to activate NF-κB remained unaltered by the TRAF2 deficiency. These data suggest that the direct binding of TRAF6 rather than TRAF3 to CD40 mediates NF-κB signaling in the absence of TRAF2.
FIG. 6.
CD40 critically depends on TRAF6 for signal transduction. (A) A dominant-negative (DN), N terminus-deleted TRAF6 but not TRAF3 mutant suppresses CD40-mediated NF-κB transcriptional activity in the absence of TRAF2. Approximately 3 × 105 TRAF2−/− CD40 fibroblasts were transiently transfected with an NF-κB-responsive luciferase reporter and a β-galactosidase plasmid in the presence or absence of increasing amounts (0, 50, or 100 ng) of dominant-negative TRAFs. Thirty-six hours later, cells were stimulated with 0.5 μg/ml CD40L for 8 h or left untreated, and the luciferase and β-galactosidase activities were measured. The relative luciferase values (RLV) represent the increase (n-fold) in the ratio of the luciferase and β-galactosidase measurements relative to untreated controls, which were given the arbitrary value of 1. Data represent mean values ± standard deviations from three independent experiments. (B) The binding of TRAF6 to CD40 mediates NF-κB signaling in TRAF2-deficient cells. TRAF2+/+ CD40 or TRAF2−/− CD40 fibroblasts were transiently transfected with an NF-κB-responsive luciferase reporter and a β-galactosidase plasmid in the presence of WT CD40, CD40mT6, or CD40mT2/T3. Cells were then stimulated with CD40L and analyzed for reporter activity as described in panel A. Data represent mean values ± standard deviations from three independent experiments. (C) Immunoblot showing the expression of TRAF6, TRAF2, and CD40 in immortalized CD40-transfected TRAF6+/+ and TRAF6−/− fibroblasts used in this study. (D) Phosphorylation of p38 and activation of JNK are impaired in CD40L-stimulated (i) but not TNF-treated (ii) TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were stimulated with CD40L for 20 or 30 min or left untreated. Lysates were immunoblotted for the phosphorylated or total p38 or immunoprecipitated with an anti-JNK1 antibody. The immunoprecipitates were examined for activity toward c-Jun as a substrate or were immunoblotted with a rabbit polyclonal JNK1 antibody. As a control, treatment with 25 ng/ml TNF induced similar levels of p38 and JNK activation in of TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts. Data are representative of at least three independent experiments. IVK, in vitro kinase assay. (E) Phosphorylation of Akt is impaired in CD40L-stimulated but not PDGF-treated TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were treated with 0.5 μg/ml CD40L for 20 or 30 min or with 50 ng/ml PDGF or were left untreated as indicated. Lysates were then immunoblotted for the phosphorylated or total Akt. (F) NF-κB binding activity is impaired in CD40L-stimulated but not TNF-treated TRAF6−/− CD40 fibroblasts. TRAF6+/+ CD40 and TRAF6−/− CD40 fibroblasts were treated with 0.5 μg/ml CD40L or 25 ng/ml TNF or were left untreated as indicated. Nuclear proteins were isolated and examined for binding to a 32P-labeled oligonucleotide probe containing the HIV-LTR NF-κB binding site. Three independent experiments were performed and gave similar results.
The binding of TRAF6 to CD40 plays only a minor role in CD40L-induced NF-κB activation (Fig. 2D and 6B). To address the overall impact of TRAF6 deficiency on CD40 signaling, fibroblast cell lines from TRAF6−/− and TRAF6+/+ mice were established as described in Materials and Methods. These cells were stably transfected with a CD40 expression vector, and positive clones expressing comparable levels of CD40 (Fig. 6C) were selected for subsequent analysis. The absence of TRAF6 in the CD40-transfected knockout fibroblasts (TRAF6−/− CD40) was confirmed by immunoblotting (Fig. 6C). As expected, however, these cells expressed TRAF2 (Fig. 6C).
TRAF6+/+ CD40 and TRAF6−/− CD40 cell cultures were stimulated with CD40L for various time intervals, and lysates were examined for the activation of Akt, JNK, and p38. The results showed that CD40 ligation efficiently engages these signaling pathways in CD40-transfected TRAF6+/+ fibroblasts. Interestingly, however, these experiments demonstrated that the CD40L-induced activation of Akt, JNK, and p38 was completely abolished in TRAF6−/− CD40 cells (Fig. 6D and E). The effects of CD40 stimulation on NF-κB activation were also evaluated in CD40-expressing TRAF6+/+ and TRAF6−/− fibroblasts by EMSA. Whereas a significant increase in the NF-κB binding activity was noted in CD40L-stimulated TRAF6+/+CD40 cells, TRAF6−/− CD40 cultures completely failed to respond (Fig. 6F).
The specificity of the observed phenomena was examined by stimulating TRAF6+/+ CD40 and TRAF6−/− CD40 cells with PDGF, a growth factor which potently activates the PI3 kinase/Akt signaling pathway (23) or with TNF-α, a cytokine that robustly engages the JNK, p38, and NF-κB cascades. The results showed normal Akt phosphorylation in PDGF-stimulated TRAF6−/− CD40 cells (Fig. 6E). Likewise, no difference in the activation of JNK, p38, and NF-κB was noted between TRAF6+/+ CD40 and TRAF6−/− CD40 cells treated with TNF-α (Fig. 6D and F).
Reconstitution experiments were then performed to confirm that the defective CD40-mediated signal transduction in TRAF6−/− CD40 fibroblasts was indeed TRAF6 dependent. In these experiments, TRAF6−/− CD40 cells were infected with a TRAF6-expressing retrovirus or control virus and either examined for expression of TRAF6 (Fig. 7A) or stimulated with CD40L and examined for the activation status of NF-κB, MAPK, and Akt (Fig. 7B). The results showed that exogenous TRAF6 restored the CD40L-induced signal transduction in TRAF6−/− CD40 cells. We conclude that TRAF6 is specifically required for CD40-mediated activation of the NF-κB, JNK, p38, and Akt signaling pathways in nonhemopoietic cells.
FIG. 7.
Expression of TRAF6 restores the CD40 signaling deficiency in TRAF6−/− CD40 fibroblasts. Cells were infected with a TRAF6 or control (CNTR) retrovirus (rv) in two successive 12-h incubations, as described in Materials and Methods. At 36 h postinfection, cells were reseeded and selected for puromycin resistance during a 2-week period. Antibiotic-resistant clones were expanded and either examined for TRAF6 expression (A) or stimulated with CD40L for 0 or 20 min and analyzed for the activation status of JNK, p38, or Akt or for the levels of endogenous IκBα by immunoblot (B). IVK, in vitro kinase assay.
TRAF6 regulates TRAF2-dependent CD40 signal transduction.
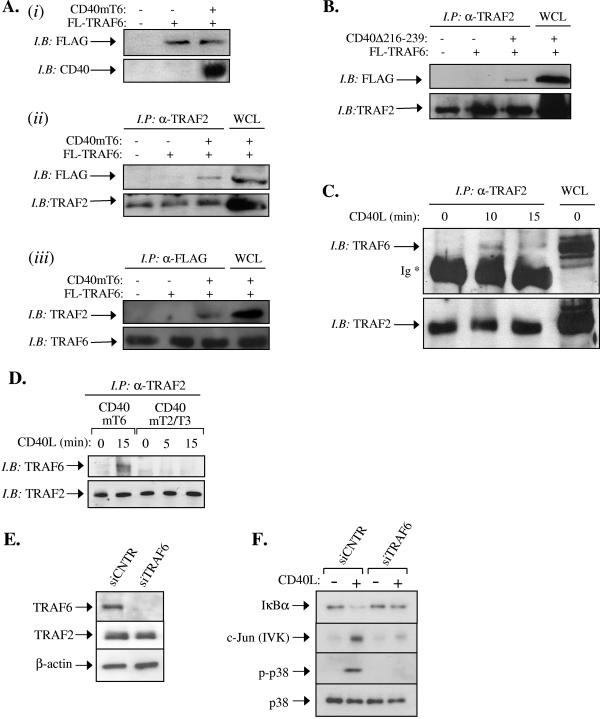
The results shown in Fig. 6 were unexpected because the TRAF6-interacting membrane-proximal CD40 domain has a minor role in CD40 signaling (Fig. 2). Moreover, no direct association of TRAF6 with CD40mT6 is detected by GST pull-down assays (Fig. 1B) (34, 56). We therefore reasoned that TRAF6 may regulate CD40 signal transduction not only through its direct binding to the established CD40 Q234 E235 core motif (56) but also indirectly via its effects downstream of the CD40/TRAF2 signaling complex, which is a key regulator of CD40 signal transduction (Fig. 2 to 4 and 6B). In line with this notion, previous studies have demonstrated that TRAF2 and TRAF6 directly interact in yeast two-hybrid assays and coimmunoprecipitate when overexpressed (6). To test if this association occurs in the context of CD40 signaling, immunoprecipitation experiments were first carried out in lysates from human embryonic kidney (HEK) 293 cells transfected with FLAG-tagged TRAF6, in the presence or absence of a CD40mT6-expression vector. Endogenous TRAF2 was immunoprecipitated and then immunoblotted with FLAG MAb (Fig. 8A ii). In other experiments FLAG-tagged TRAF6 was immunoprecipitated with anti-FLAG and then immunoblotted with a TRAF2 polyclonal antibody (Fig. 8Aiii). The results of these analyses showed that, whereas TRAF2 weakly associates with overexpressed TRAF6, this interaction is significantly augmented in the presence of CD40mT6, the mutated CD40 that does not directly bind TRAF6 (Fig. 8A). Similar results were obtained when cells were transfected with the deletion mutant CD40Δ216-239 (a deletion of residues 216 to 239) (16) which lacks the TRAF6 binding domain of CD40 (Fig. 8B).
FIG. 8.
TRAF6 regulates TRAF2-dependent CD40-mediated signaling. (A) TRAF2 interacts with exogenously expressed TRAF6 in the presence of CD40mT6. HEK 293 cells were transiently transfected with FLAG-tagged TRAF6 in the presence or absence of CD40mT6, and 24 h later cells were lysed. Lysates (50 μg) were immunoblotted with anti-Flag or anti-CD40 to confirm expression of transfected cDNAs (i). Lysates (750 μg) were also immunoprecipitated with anti-TRAF2 MAb and immunoblotted with either anti-FLAG or anti-TRAF2 polyclonal antibody, in parallel with 50 μg of whole-cell lysate (ii), or immunoprecipitated with anti-FLAG MAb and immunoblotted with either anti-TRAF2 or anti-TRAF6 polyclonal antibody, in parallel with 50 μg of whole-cell lysate (iii). (B) TRAF2 interacts with exogenously expressed TRAF6 in the presence of CD40Δ(216-239). HEK 293 cells were transiently transfected with FLAG-tagged TRAF6 in the presence or absence of CD40Δ(216-239), and 24 h later cells were lysed. Lysates (750 μg) were immunoprecipitated with anti-TRAF2 MAb and immunoblotted with either anti-FLAG or anti-TRAF2 polyclonal antibody, in parallel with 50 μg of whole-cell lysate. (C) TRAF6 and TRAF2 coimmunoprecipitate in CD40L-stimulated carcinoma cells carrying a mutated CD40 that does not directly bind TRAF6. HeLa-CD40mT6 cells were treated with 0.1 μg/ml CD40L for various time intervals or left untreated, as indicated. Lysates were then immunoprecipitated with an anti-TRAF2 MAb and immunoblotted with either anti-TRAF6 or anti-TRAF2 polyclonal antibody. Similar results were obtained in two additional independent experiments. Ig, immunoglobulin heavy chain. (D) TRAF6 and TRAF2 do not coimmunoprecipitate in CD40L-stimulated carcinoma cells carrying a mutated CD40 that does not directly bind TRAF2/TRAF3. HeLa-CD40mT2/T3 (lanes 3 to 5) or, as a control, HeLa-CD40mT6 (lanes 1 to 2) cells were treated with 0.1 μg/ml CD40L for various time intervals or left untreated, as indicated. Lysates were then immunoprecipitated with an anti-TRAF2 MAb and immunoblotted with either anti-TRAF6 or anti-TRAF2 polyclonal antibody. (E) HeLa-CD40mT6 cells were transfected with siRNA targeting TRAF6 or the unrelated luciferase gene product. Lysates were analyzed for TRAF6, TRAF2, or β-actin levels by immunoblotting. (F) RNAi-mediated knock-down of TRAF6 expression results in diminished CD40-induced NF-κB, JNK, and p38 activation in HeLa-CD40mT6 epithelial cells. Following transfection with TRAF6 or luciferase siRNA, HeLa-CD40mT6 cells were stimulated with 0.1 μg/ml CD40L or left untreated, as indicated. Lysates were isolated and analyzed for the expression of IκBα and of the phosphorylation and activation of p38 and JNK, respectively. Data are representative of three independent experiments. IP, immunoprecipitate; IB, immunoblot; WCL, whole-cell lysate.
In the experiments described above, CD40mT6 was transiently overexpressed, a process known to result in signal activation through the transient formation of receptor multimers and recruitment of TRAFs in a ligand-independent manner (7, 49, 51). In contrast, the stable expression of CD40 in HeLa cells does not induce elevated signal transduction (Fig. 2), indicating the presence of “diffused” CD40 monomers at the plasma membrane and the requirement of CD40L-induced oligomerization for signal initiation. We, therefore, determined whether the association of TRAF2 with TRAF6 can also be observed with endogenous proteins in HeLa-CD40mT6 cells stimulated with recombinant soluble CD40L. To this end, lysates were prepared from CD40L-stimulated or control untreated cells, immunoprecipitated with a mouse TRAF2 MAb, and immunoblotted with anti-TRAF6 polyclonal antibody. Whereas no detectable association of endogenous TRAF6 and TRAF2 was observed in the absence of stimulation, CD40 ligation reproducibly promoted their interaction in a time-dependent manner (Fig. 8C). Interestingly, no association of TRAF2 and TRAF6 was observed upon stimulation of HeLa-CD40mT2/T3 cells with CD40L (Fig. 8D). We conclude that TRAF2 and TRAF6 associate following CD40 activation and that this interaction occurs independently of the TRAF6 binding to CD40. Attempts to coprecipitate CD40mT6 and TRAF6 in HeLa-CD40mT6 and HEK 293 cells were, however, unsuccessful. This may indicate that the CD40/TRAF2/TRAF6 complex is unstable or may reflect technical limitations in protein detection as only a portion of endogenous TRAF2 interacts with CD40 (58) and, in turn, only a portion of TRAF6 coprecipitates with TRAF2 (Fig. 8A and C). An alternative possibility is that following CD40 ligation, TRAF6 is not recruited to a CD40/TRAF2-containing complex at the plasma membrane but forms cytoplasmic complexes with TRAF2.
To provide further evidence that the observed TRAF2-TRAF6 association is functional, TRAF6 was knocked down by RNAi in HeLa-CD40mT6 cells. Immunoblot analysis confirmed significant decrease of TRAF6 levels in these cells, whereas transfection with an unrelated siRNA targeting luciferase had no effect (Fig. 8E). As expected, the levels of TRAF2 were not influenced by either of these treatments (Fig. 8E). Parallel cultures were stimulated with CD40L and analyzed for the activation status of JNK, p38, and the expression of IκBα. The results showed a severe reduction in the catalytic activity of JNK and the phosphorylation of p38 in HeLa-CD40mT6 cells transfected with TRAF6 siRNA (Fig. 8F). In addition, the CD40-mediated degradation of IκBα was greatly attenuated in these cultures (Fig. 8F). Therefore, TRAF6 associates with and modulates the function of TRAF2 in response to CD40 activation.
DISCUSSION
The emerging role of the CD40 pathway in disease pathogenesis and cancer therapy necessitates the analysis of CD40 signal transduction and its regulation by TRAF proteins in a wide range of tissue types. This is corroborated by the observation that CD40 and TRAFs are functionally expressed not only in lymphoid cells but also in nonhemopoietic lineages (21, 62) and by the distinct role of TRAF2 in CD40-induced NF-κB activation in normal versus malignant B lymphocytes (27, 31, 45). Here, we have addressed for the first time the physiological contribution of TRAF2 and TRAF6 to CD40 signaling in nonhemopoietic cells by utilizing RNA interference and knockout approaches. These studies have been complemented by the analysis of the signaling capacity of a series of mutated CD40 receptors expressed in carcinoma cell lines and fibroblasts. These molecules carry point mutations in the cytoplasmic tail of CD40 which specifically abolish binding of TRAF2 and TRAF3, of TRAF6, or of all three TRAF proteins (Fig. 1). Of note, we have demonstrated that the introduction of a Q263→A mutation in the cytoplasmic C terminus of CD40 exclusively disrupts the binding of TRAF3, thus allowing for the first time the evaluation of the specific contribution of the TRAF2-CD40 interaction to CD40L-induced signaling.
Our results demonstrate a major role for TRAF2 in CD40 signal transduction and function in nonhemopoietic cells. Thus, the CD40-mediated activation of the IκBα/NF-κB pathway is severely affected by mutations in the CD40 cytoplasmic tail which disrupt its association with TRAF2. Conversely, a mutated CD40 capable only of binding TRAF2 (CD40mT3/T6) maintains much of the NF-κB-inducing properties of wild-type CD40 (Fig. 2D). Moreover, we have shown that the suppression or elimination of TRAF2 expression in epithelial cell lines and fibroblasts significantly impairs, but does not abolish, CD40L-induced IκBα degradation and NF-κB binding activity (Fig. 4D and E). The importance of TRAF2 in CD40-induced NF-κB activation in nonhemopoietic cells is reminiscent of its reported major role in NF-κB signaling in normal B lymphocytes (27, 40). In contrast, however, Bishop et al. showed that TRAF2 deficiency in B-cell lymphoma lines has no impact on CD40-mediated NF-κB activation (31), suggesting that the wiring of CD40 signal transduction in these cells differs from normal mouse B lymphocytes (27, 40), fibroblasts, and human epithelial cells (this study). These findings also raise the possibility that the levels of TRAF protein expression and/or associated molecules may control CD40 signal transduction in different cell types, a hypothesis that merits further investigation. In addition to NF-κB, we have demonstrated that TRAF2 plays a major physiological role in the CD40-mediated activation of p38 and Akt and, to a lesser degree, JNK signals (Fig. 2 to 4). Given the established role of these pathways in transcriptional and translational control (11, 12, 25), a plethora of CD40-mediated functions involved in the regulation of cell growth, survival, and cytokine gene expression may largely depend on TRAF2.
Theoretically, redundancy between TRAF2 and TRAF3 in CD40-mediated signal transduction may exist. However, a number of observations argue against this possibility. First, engagement of a mutated CD40 receptor capable only of binding TRAF2 and TRAF3 in TRAF2−/− fibroblasts fails to activate NF-κB (Fig. 6B). Second, expression of a dominant-negative TRAF3 has no impact on CD40-induced NF-κB transactivation in TRAF2−/− fibroblasts, whereas a dominant-negative TRAF6 eliminates NF-κB signaling in these cells (Fig. 6A). Third, unlike TRAF2, overexpression of TRAF3 does not activate NF-κB (33). These findings, coupled with the observation that a mutated CD40 which only binds TRAF2 (CD40mT3/T6) maintains much of the signaling properties of wild-type CD40 (Fig. 2), suggest that TRAF2 provides the major vehicle for CD40 signal transduction in nonhemopoietic cells.
While TRAF2 is an important component of CD40 signal transduction in nonhemopoietic cells, data shown in the present study demonstrate that the CD40L-mediated activation of NF-κB, JNK, p38, and Akt strictly requires the integrity of TRAF6 (Fig. 6). This finding was unexpected, given that the TRAF6-interacting membrane-proximal domain of CD40 has a minor role in signal transduction (Fig. 2), and suggested that TRAF6 can also function downstream of the CD40/TRAF2 signaling complex. The E3 ubiquitin ligase activity of TRAF6 (13) could, theoretically, result in increased TRAF2 protein turnover in TRAF6−/− CD40 cells and, thereby, diminished CD40 signal transduction. However, we observed no change in TRAF2 levels in TRAF6−/− CD40 fibroblasts stimulated for up to 30 min with CD40L (data not shown). In support of this finding, the TRAF6 ablation did not have impact on TNF-induced NF-κB, p38, and JNK activation (Fig. 6D and F) which also largely depends on the integrity of TRAF2 (15, 60).
TRAF molecules are known to form homo- and heterodimers via their C-terminal TRAF-C domains (9). A previous report using the yeast two-hybrid assay and coimmunoprecipitations in mammalian cells showed that TRAF6 directly interacts with TRAF2 (6). A more recent study has demonstrated that TRAF6 is recruited to a TRAF2-containing signaling complex induced by the Epstein-Barr virus-encoded latent membrane protein 1 (52). Our data demonstrate that TRAF6 associates with TRAF2 in CD40L-stimulated cells carrying mutated receptors that do not directly bind TRAF6 (Fig. 8A to C). Moreover, this interaction was shown to be functional because the RNAi-mediated knock-down of TRAF6 impaired the signaling capacity of this mutated receptor (Fig. 8F). Interestingly, however, we have been unable to observe association of TRAF6 with TRAF2 upon activation of CD40mT2/T3. This finding suggests that the inducible TRAF2-TRAF6 interaction is exclusively mediated by the TRAF2/TRAF3- but not the TRAF6-binding domain of CD40. Therefore, significant differences exist in the mechanisms of signal transduction triggered by the two TRAF-interacting domains of CD40. These differences may be the result of the relative affinities of various TRAF molecules for CD40. Indeed, CD40 only weakly binds TRAF6 (37, 38), and amplification of this interaction significantly impacts on the functional consequences of CD40 stimulation (26, 35). It is also possible that the TRAF2-TRAF6 association is regulated by proteins that are selectively recruited to CD40-bound TRAF2 but not CD40-bound TRAF6. TRAF1, cIAP1, and cIAP2, for example, are specifically recruited to CD40 through TRAF2 (22). However, it is unlikely that TRAF1 is the protein that modulates the TRAF2-TRAF6 interaction because interference with TRAF1 levels does not have a major impact on CD40L-induced NF-κB and JNK signaling (19, 55). Alternatively, the association of TRAF2 with TRAF6 may require TRAF6 epitopes that are masked by its direct binding to CD40. Consistent with this possibility, recent crystallographic analysis has revealed marked differences in the conformation of the CD40/TRAF6 versus CD40/TRAF2 complex (59).
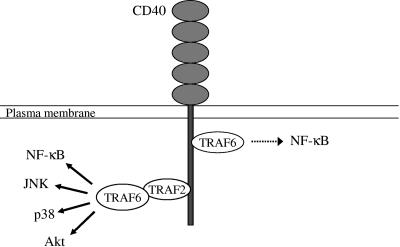
On the basis of the findings shown in the present study, we propose a model, illustrated in Fig. 9, of CD40 signaling in nonhemopoietic cells. Following CD40 stimulation, TRAF6 and TRAF2 are directly recruited to two distinct domains in the cytoplasmic tail of CD40 and mediate signal transduction. The direct binding of TRAF6 only partly contributes to the activation of NF-κB, whereas the binding of TRAF2 plays a major role in CD40 ligand-induced signaling. However, TRAF6 also associates with TRAF2 downstream of the CD40/TRAF2 signaling complex and regulates its function. We propose that the TRAF2-TRAF6 interaction is required for the recruitment and activation of kinases responsible for the engagement of the NF-κB, JNK, p38, and Akt pathways. A recent study has shown that germinal center kinase associates with both TRAF2 and TRAF6 and mediates JNK activation in a TRAF6-dependent manner (64). Therefore, germinal center kinase may represent an active component of the TRAF2/TRAF6 signaling complex that regulates JNK in response to CD40 ligation. Taken together, the data shown in the present study provide definitive evidence for the physiological roles of TRAF2 and TRAF6 in CD40 signaling in nonhemopoietic cells and shed new light into the multiple roles played by TRAF6 in CD40 signal transduction.
FIG. 9.
The role of TRAF2 and TRAF6 in CD40-mediated signaling: a proposed model. Based on the data shown in the present study, we propose that the direct binding of TRAF6 only partly contributes to the activation of NF-κB (dotted arrow), whereas the binding of TRAF2 plays a major role in CD40L-induced signal transduction (solid arrows). TRAF6 also associates with TRAF2 and controls the function of the CD40/TRAF2 signaling complex. We propose that the TRAF2-TRAF6 interaction is required for the recruitment of kinases responsible for the engagement of the NF-κB, JNK, p38, and Akt pathways.
Supplementary Material
Acknowledgments
We thank Liz Hodgkins for technical assistance.
This work was supported by a Cancer Research UK grant to A.G.E. and L.S.Y. and a Medical Research Council (London, United Kingdom) Career Development Award to A.G.E.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adawi, A., Y. Zhang, R. Baggs, P. Rubin, J. Williams, J. Finkelstein, and R. P. Phipps. 1998. Blockade of CD40-CD40 ligand interactions protects against radiation-induced pulmonary inflammation and fibrosis. Clin. Immunol. Immunopathol. 89:222-230. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen, C., E. Manning, L. D. Erickson, B. O'Connor, E. F. Lind, S. S. Pullen, M. R. Kehry, and R. J. Noelle. 2002. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat Immunol. 3:451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andjelic, S., C. Hsia, H. Suzuki, T. Kadowaki, S. Koyasu, and H. C. Liou. 2000. Phosphatidylinositol 3-kinase and NF-kappa B/Rel are at the divergence of CD40-mediated proliferation and survival pathways. J. Immunol. 165:3860-3867. [DOI] [PubMed] [Google Scholar]
- 4.Baud, V., Z.-G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 13:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 153:4357-4366. [PubMed] [Google Scholar]
- 6.Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D. V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature (London) 383:443-446. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, G., and D. Baltimore. 1996. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 10:963-973. [DOI] [PubMed] [Google Scholar]
- 8.Cho, C. S., M. L. Cho, S. Y. Min, W. U. Kim, D. J. Min, S. S. Lee, S. H. Park, J. Choe, and H. Y. Kim. 2000. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. J. Immunol. 164:5055-5061. [DOI] [PubMed] [Google Scholar]
- 9.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 10.Davies, C. C., D. Bem, L. S. Young, and A. G. Eliopoulos. 2005. NF-kappaB overrides the apoptotic program of TNF receptor 1 but not CD40 in carcinoma cells. Cell Signal. 17:729-738. [DOI] [PubMed] [Google Scholar]
- 11.Davies, C. C., J. Mason, M. J. Wakelam, L. S. Young, and A. G. Eliopoulos. 2004. Inhibition of phosphatidylinositol 3-kinase- and ERK MAPK-regulated protein synthesis reveals the pro-apoptotic properties of CD40 ligation in carcinoma cells. J. Biol. Chem. 279:1010-1019. [DOI] [PubMed] [Google Scholar]
- 12.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 13.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 14.Desvignes, C., F. Esteves, N. Etchart, C. Bella, C. Czerkinsky, and D. Kaiserlian. 1998. The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo. Clin. Exp. Immunol. 113:386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devin, A., Y. Lin, and Z. G. Liu. 2003. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 4:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliopoulos, A. G., C. Davies, P. G. Knox, N. J. Gallagher, S. C. Afford, D. H. Adams, and L. S. Young. 2000. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol. Cell. Biol. 20:5503-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eliopoulos, A. G., C. W. Dawson, G. Mosialos, J. E. Floettmann, M. Rowe, R. J. Armitage, J. Dawson, J. M. Zapata, D. J. Kerr, M. J. O. Wakelam, J. C. Reed, E. Kieff, and L. S. Young. 1996. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 13:2243-2254. [PubMed] [Google Scholar]
- 18.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kB pathway involving TNF receptor-associated factors. Oncogene 14:2899-2916. [DOI] [PubMed] [Google Scholar]
- 19.Eliopoulos, A. G., E. R. Waites, S. M. Blake, C. Davies, P. Murray, and L. S. Young. 2003. TRAF1 is a critical regulator of JNK signaling by the TRAF-binding domain of the Epstein-Barr virus-encoded latent infection membrane protein 1 but not CD40. J. Virol. 77:1316-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliopoulos, A. G., C. C. Wang, C. D. Dumitru, and P. N. Tsichlis. 2003. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 22:3855-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eliopoulos, A. G., and L. S. Young. 2004. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr. Opin. Pharmacol. 4:360-367. [DOI] [PubMed] [Google Scholar]
- 22.Fotin-Mleczek, M., F. Henkler, A. Hausser, H. Glauner, D. Samel, A. Graness, P. Scheurich, D. Mauri, and H. Wajant. 2004. Tumor necrosis factor receptor-associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-kappaB activation. J. Biol. Chem. 279:677-685. [DOI] [PubMed] [Google Scholar]
- 23.Franke, T. F., S.-I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 24.Ghamande, S., B. L. Hylander, E. Oflazoglu, S. Lele, W. Fanslow, and E. A. Repasky. 2001. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 61:7556-7562. [PubMed] [Google Scholar]
- 25.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 26.Gohda, J., T. Akiyama, T. Koga, H. Takayanagi, S. Tanaka, and J. Inoue. 2005. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 24:790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grech, A. P., M. Amesbury, T. Chan, S. Gardam, A. Basten, and R. Brink. 2004. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity 21:629-642. [DOI] [PubMed] [Google Scholar]
- 28.Hess, S., and H. Engelmann. 1996. A novel function of CD40: induction of cell death in transformed cells. J. Exp. Med. 183:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill, S. C., S. J. Youde, S. Man, G. R. Teale, A. J. Baxendale, A. Hislop, C. C. Davies, D. M. Luesley, A. M. Blom, A. B. Rickinson, L. S. Young, and A. G. Eliopoulos. 2005. Activation of CD40 in cervical carcinoma cells facilitates CTL responses and augments chemotherapy-induced apoptosis. J. Immunol. 174:41-50. [DOI] [PubMed] [Google Scholar]
- 30.Hirano, A., D. L. Longo, D. D. Taub, D. K. Ferris, L. S. Young, A. G. Eliopoulos, A. Agathanggelou, N. Cullen, J. Macartney, W. C. Fanslow, and W. J. Murphy. 1999. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood 93:2999-3007. [PubMed] [Google Scholar]
- 31.Hostager, B. S., S. A. Haxhinasto, S. L. Rowland, and G. A. Bishop. 2003. Tumor necrosis factor receptor-associated factor 2 (TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J. Biol. Chem. 278:45382-45390. [DOI] [PubMed] [Google Scholar]
- 32.Hu, H. M., K. O'Rourke, M. S. Boguski, and V. M. Dixit. 1994. Novel ring finger protein interacts with the cytoplasmic domain of CD40. J. Biol. Chem. 269:30069-30072. [PubMed] [Google Scholar]
- 33.Ishida, T. K., T. Tojo, T. Aoki, N. Kobayashi, T. Ohishi, T. Watanabe, T. Yamamoto, and J. Inoue. 1996. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc. Natl. Acad. Sci. USA 93:9437-9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabara, H., D. Laouini, E. Tsitsikov, E. Mizoguchi, A. Bhan, E. Castigli, F. Dedeoglu, V. Pivniouk, S. Brodeur, and R. Geha. 2002. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity 17:265-276. [DOI] [PubMed] [Google Scholar]
- 35.Kadono, Y., F. Okada, C. Perchonock, H. D. Jang, S. Y. Lee, N. Kim, and Y. Choi. 2005. Strength of TRAF6 signalling determines osteoclastogenesis. EMBO Rep. 6:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi, T., P. T. Walsh, M. C. Walsh, K. M. Speirs, E. Chiffoleau, C. G. King, W. W. Hancock, J. Caamano, C. A. Hunter, P. Scott, L. A. Turka, and Y. Choi. 2003. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19:353-363. [DOI] [PubMed] [Google Scholar]
- 37.Lee, H. H., P. W. Dempsey, T. P. Parks, X. Zhu, D. Baltimore, and G. Cheng. 1999. Specificities of CD40 signaling: involvement of TRAF2 in CD40-induced NF-κB activation and intercellular adhesion molecule-1 up-regulation. Proc. Natl. Acad. Sci. USA 96:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leo, E., K. Welsh, S.-I. Matsuzawa, J. M. Zapata, S. Kitada, R. S. Mitchell, K. R. Ely, and J. C. Reed. 1999. Differential requirements for tumor necrosis factor receptor-associated factor family proteins in CD40-mediated induction of NF-κB and Jun N-terminal kinase activation. J. Biol. Chem. 274:22414-22422. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y. Y., M. Baccam, S. B. Waters, J. E. Pessin, G. A. Bishop, and G. A. Koretzky. 1996. CD40 ligation results in protein kinase C-independent activation of ERK and JNK in resting murine splenic B cells. J. Immunol. 157:1440-1447. [PubMed] [Google Scholar]
- 40.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mak, T. W., and W. C. Yeh. 2002. Signaling for survival and apoptosis in the immune system. Arthritis Res. 4(Suppl. 3):S243-S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehling, A., K. Loser, G. Varga, D. Metze, T. A. Luger, T. Schwarz, S. Grabbe, and S. Beissert. 2001. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J Exp. Med. 194:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano, H., S. Sakon, H. Koseki, T. Takemori, K. Tada, M. Matsumoto, E. Munechika, T. Sakai, T. Shirasawa, H. Akiba, T. Kobata, S. M. Santee, C. F. Ware, P. D. Rennert, M. Taniguchi, H. Yagita, and K. Okumura. 1999. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc. Natl. Acad. Sci. USA 96:9803-9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natoli, G., A. Costanzo, A. Ianni, D. J. Templeton, J. R. Woodgett, C. Balsano, and M. Levrerop. 1997. Activation of SAPK/JNK by TNF Receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200-203. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen, L. T., G. S. Duncan, C. Mirtsos, M. Ng, D. E. Speiser, A. Shahinian, M. W. Marino, T. W. Mak, P. S. Ohashi, and W.-C. Yeh. 1999. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity 11:379-389. [DOI] [PubMed] [Google Scholar]
- 46.Ni, C. Z., K. Welsh, E. Leo, C. K. Chiou, H. Wu, J. C. Reed, and K. R. Ely. 2000. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. USA 97:10395-10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noelle, R. J. 1996. CD40 and its ligand in host defense. Immunity 4:415-419. [DOI] [PubMed] [Google Scholar]
- 48.Peguet-Navarro, J., C. Dalbiez-Gauthier, C. Moulon, O. Berthier, A. Reano, M. Gaucherand, J. Banchereau, F. Rousset, and D. Schmitt. 1997. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J. Immunol. 158:144-152. [PubMed] [Google Scholar]
- 49.Pullen, S. S., M. E. Labadia, R. H. Ingraham, S. M. McWhirter, D. S. Everdeen, T. Alber, J. J. Crute, and M. R. Kehry. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 38:10168-10177. [DOI] [PubMed] [Google Scholar]
- 50.Rissoan, M. C., C. Van Kooten, P. Chomarat, L. Galibert, I. Durand, F. Thivolet-Bejui, P. Miossec, and J. Banchereau. 1996. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin. Exp. Immunol. 106:481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothe, M., V. Sarma, V. M. Dixit, and D. V. Goeddel. 1995. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269:1424-1427. [DOI] [PubMed] [Google Scholar]
- 52.Schultheiss, U., S. Puschner, E. Kremmer, T. W. Mak, H. Engelmann, W. Hammerschmidt, and A. Kieser. 2001. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20:5678-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamenkovic, I., E. A. Clark, and B. Seed. 1989. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 8:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong, A. W., and M. J. Stone. 2003. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 10:1-13. [DOI] [PubMed] [Google Scholar]
- 55.Tsitsikov, E. N., D. Laouini, I. F. Dunn, T. Y. Sannikova, L. Davidson, F. W. Alt, and R. S. Geha. 2001. TRAF1 is a negative regulator of TNF signaling. Enhanced TNF signaling in TRAF1-deficient mice. Immunity 15:647-657. [DOI] [PubMed] [Google Scholar]
- 56.Tsukamoto, N., N. Kobayashi, S. Azuma, T. Yamamoto, and J. Inoue. 1999. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA 96:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 58.Xie, P., B. S. Hostager, and G. A. Bishop. 2004. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye, H., J. R. Arron, B. Lamothe, M. Cirilli, T. Kobayashi, N. K. Shevde, D. Segal, O. K. Dzivenu, M. Vologodskaia, M. Yim, K. Du, S. Singh, J. W. Pike, B. G. Darnay, Y. Choi, and H. Wu. 2002. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418:443-447. [DOI] [PubMed] [Google Scholar]
- 60.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 61.Young, L. S., C. W. Dawson, K. W. Brown, and A. B. Rickinson. 1989. Identification of a human epithelial cell surface protein sharing an epitope with C3d/Epstein-Barr virus receptor molecule of B lymphocytes. Int. J. Cancer 43:786-794. [DOI] [PubMed] [Google Scholar]
- 62.Zapata, J. M., M. Krajewska, S. Krajewski, S. Kitada, K. Welsh, A. Monks, N. McCloskey, J. Gordon, T. J. Kipps, R. D. Gascoyne, A. Shabaik, and J. C. Reed. 2000. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J. Immunol. 165:5084-5096. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, Y., H. J. Cao, B. Graf, H. Meekins, T. J. Smith, and R. P. Phipps. 1998. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J. Immunol. 160:1053-1057. [PubMed] [Google Scholar]
- 64.Zhong, J., and J. M. Kyriakis. 2004. Germinal center kinase is required for optimal Jun N-terminal kinase activation by Toll-like receptor agonists and is regulated by the ubiquitin proteasome system and agonist-induced, TRAF6-dependent stabilization. Mol. Cell. Biol. 24:9165-9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.