Chlamydia trachomatis sexually transmitted infections cause considerable morbidity and socioeconomic burden worldwide, despite significant advances in our understanding of the biology (29, 31, 57), pathogenesis (11, 83, 117), genomics (94), and epidemiology (91) of this parasite. Chlamydial urogenital tract infections are readily cured with antibiotics, but control measures based on antimicrobial chemotherapy alone are hampered by the frequency of asymptomatic infections and delayed diagnosis (9). Definitive control of C. trachomatis sexually transmitted diseases (STDs) is possible through the development of a safe and efficacious vaccine (24). Progress toward the development of an effective vaccine has been disappointingly modest, as it has been for vaccines to other sexually transmitted pathogens that infect the genital tract mucosae. The strict tropism for mucosal epithelial cells, the complex biology and antigenic structure, and the predilection to cause persistent infection have presented formidable challenges to chlamydial vaccine development. A heightened understanding of protective immunity to C. trachomatis urogenital infection has emerged in the past decade from studies using a mouse model of chlamydial genital tract infection. The insights are of considerable interest because they offer promise for the development of an efficacious chlamydial vaccine. This review focuses on that progress and summarizes the current understanding of protective immune mechanisms that function against murine chlamydial urogenital infection. We also discuss specific requirements for a vaccine to protect against chlamydial STDs and the challenges presently confronting us in achieving that goal.
C. TRACHOMATIS AND SEXUALLY TRANSMITTED DISEASE
The genus Chlamydia comprises a group of obligate intracellular bacterial pathogens that are characterized by a unique biphasic developmental cycle (29). Infection is initiated by the attachment of the small (200 nm), infectious, metabolically inert elementary body (EB), which subsequently enters cells within a membrane-bound vesicle. The endocytosed, vesicle-bound EB (termed an inclusion) evades fusion with host lysosomes and rapidly differentiates into a metabolically active reticulate body (RB) that replicates by binary fission within the protected environment of the nonfusogenic inclusion. Following several rounds of cell division, the RBs reorganize and form infectious EBs. This process involves histone-mediated condensation of genomic DNA and disulfide-mediated cross-linking of chlamydial outer membrane proteins (29).
There are four commonly recognized species of Chlamydia: C. trachomatis, C. psittaci, C. pneumoniae, and C. pecorum. C. psittaci and C. pecorum are pathogens of birds and lower animals, with humans being only ancillary hosts. C. pneumoniae and C. trachomatis are human pathogens. C. pneumoniae causes acute respiratory infections and has been associated with cardiovascular disease (28). C. trachomatis is a strict pathogen of oculogenital epithelial cells. It is the etiologic agent of trachoma and is the leading cause of bacterial STDs worldwide (111, 118). C. trachomatis isolates consist of 15 major serovariants and the closely related murine strain designated MoPn (for mouse pneumonitis), which was recently reclassified as C. muridarum (26). Serovars A, B, Ba, and C cause trachoma, and serovars D to K and L1 to L3 cause sexually transmitted infections. Serovars L1, L2, and L3 cause lymphogranuloma venereum (LGV), whereas serovars D to K cause cervicitis, urethritis, and the associated complications of more severe disease such as endometritis, salpingitis, and pelvic inflammatory disease (PID) in women. The LGV strains are biologically and pathologically distinct from the D to K serovars. LGV strains transiently infect epithelial cells and then invade the submucosae to infect macrophages, which facilitates the dissemination of the infection to regional draining lymph nodes (91). In contrast, serovars D to K are noninvasive, causing infection and disease that are restricted to the urogenital mucosae. It is estimated by the World Health Organization that 90 million of the 500 million new cases of STDs per year are caused by C. trachomatis serovars D to K (118). In the United States, approximately 4 million new cases of chlamydial STDs are reported annually, and costs associated with management of these infections and associated complications exceed $2 billion (41). Moreover, chlamydial infection is associated with an increased risk of human immunodeficiency virus-related AIDS and cervical dysplasia, thereby heightening demand for development of more effective prevention measures (2, 66).
Females are particularly at risk because of their propensity to develop postinfection complications, and it is for this population that preventive measures are urgently needed. Approaches presently being studied for the management and control of chlamydial STDs in females include (i) behavioral intervention; (ii) enhanced surveillance, rapid diagnosis, and early antimicrobial treatment; (iii) topical microbicides; and (iv) immune intervention. Each of these approaches has merit and is capable of limiting infection transmission or reducing infection-related complications. However, sustained control will be achieved only by the development of an effective vaccine. The development of an efficacious vaccine against C. trachomatis genital tract infection will be facilitated by the use of animal models that closely mimic human infection and that are suitable for comprehensive immunological analyses and vaccine testing.
MURINE MODEL OF C. TRACHOMATIS GENITAL TRACT INFECTION
Genital tract infection of mice with C. trachomatis (MoPn) closely mimics, in many aspects, acute genital tract infection of women. Intravaginal inoculation produces a self-limiting infection that originates in the vaginal epithelium and ascends along the epithelial surface of the uterine horns and oviducts (61). Mice naturally resolve infection without antimicrobial chemotherapy in approximately 4 weeks and develop long-lived adaptive immunity that protects against reinfection (Fig. 1) (4, 61). The initial inflammatory response elicited by infection is characterized by a marked mucosal and submucosal infiltration of polymorphonuclear neutrophils. Lymphocytes and macrophages infiltrate the submucosae as infection resolves (Fig. 2) (61, 62, 99). Infiltrating lymphocyte subpopulations include B cells, CD4+ T cells and CD8+ T cells (Fig. 3) (62). CD4+ T cells predominate throughout the course of infection (49, 62) and small clusters of CD4+ T cells remain scattered throughout the genital tract submucosae following the resolution of infection (44, 62). Infection is confined to the genital tract mucosal epithelium (Fig. 2D) (61).
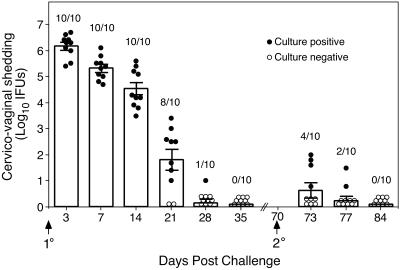
FIG. 1.
C. trachomatis MoPn genital tract infection of female C57BL/6 mice. Mice were infected intravaginally with 100 50% infectious doses (approximately 103 inclusion-forming units [IFUs]) of MoPn EBs at day 0, and IFUs were enumerated from swabs of the vaginal vault at the indicated times post primary and secondary infectious challenge. Data are presented as the mean numbers of IFUs recovered from all mice at each time point (indicated by the bar graph) and the standard errors of the means. Circles, the number of IFUs recovered from individual mice; closed circles, culture-positive mice; open circles, culture-negative animals. Values above the bars indicate the numbers of culture-positive animals per total number infected at each time point post-primary and -secondary challenge. Following the resolution of primary infection only 4 of 10 (40%) mice were infectable upon secondary challenge, thus demonstrating that solid immunity develops in the vast majority of mice. Furthermore, mice that were reinfected showed marked diminution of bacterial load and a vastly shortened course of infection. The duration of infection was 7 days or less (compared to 4 to 5 weeks for primary infection) and there was a 5.5 log10 reduction in recoverable IFUs in culture-positive reinfected mice (3 days post-secondary challenge compared to 3 days post-primary challenge).
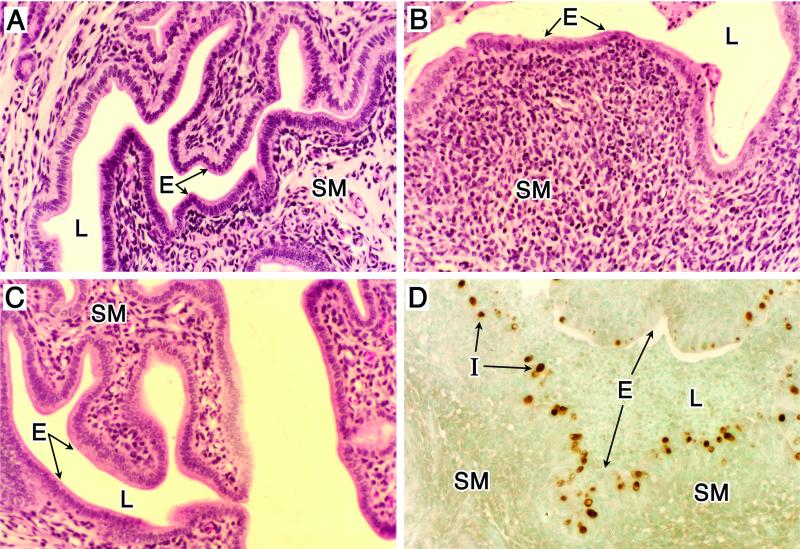
FIG. 2.
Histopathology of genital tract tissue of C57BL/6 female mice following infection with C. trachomatis (MoPn). Animals were infected as described in the legend for Fig. 1. (A to C) Uterine tissues stained with hematoxylin and eosin. (A) Noninfected. (B) Day 14 postinfection. (C) Day 42 postinfection. Note the marked thinning of the epithelial surface accompanied by an intense subacute inflammatory response consisting of polymorphonuclear cells, macrophages, and lymphocytes (B). The inflammatory response subsides as infection resolves (C). Localization of chlamydial inclusions to the epithelium was demonstrated by staining uterine tissue, obtained from mice at day 7 postinfection, with anti-MoPn MOMP immunoperoxidase (D). E, epithelium; L, lumen; I, chlamydial inclusion; SM, submucosae. Magnifications, ×100. This figure was adapted with permission from reference 61.
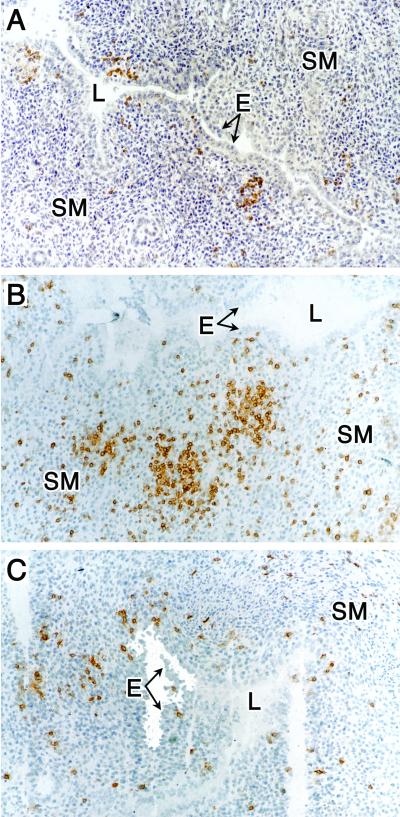
FIG. 3.
Immunohistochemical staining of B cells and T-cell subpopulations in uterine tissue from chlamydiae-infected mice at 14 days postinfection. (A) Anti-CD45R (B cells). (B) Anti-CD4 (CD4+ T cells). (C) Anti-CD8 (CD8+ T cells). Magnification, ×100. See the legend to Fig. 2 for abbreviations. This figure was adapted with permission from reference 62.
Typically, >60% of mice that resolve primary infection are resistant to reinfection with the homologous chlamydial strain and mice that are reinfected have secondary infections that are significantly shorter (7 versus 35 days) and shed far fewer infectious bacteria (>105 reduction in bacterial shedding) (Fig. 1) (61, 99). Mice that are infected following rechallenge have a mild transient inflammatory response, which is restricted to the lower genital tract. Hydrosalpinx accompanied by infertility, a complication of infection that is also observed in humans, is a common postinfection sequela (23, 61, 105, 106). Also analogous to human infection are the differences in the course of infection, antichlamydial immune responses, and disease pathology that are exhibited upon infection of different strains of mice (20, 22, 23). Thus, the pathogenesis of murine C. trachomatis genital tract infection is remarkably similar to acute infection of the human female genital tract. This similarity in pathogenesis and the strong adaptive immune response generated following the resolution of infection establish the usefulness of the model for the study of protective immunity and vaccine development. A caveat of the model is that the MoPn strain is not a naturally occurring human pathogen. The genomes of MoPn and serovar D (human isolate) are remarkably similar in gene content and order (87, 95), except for a region termed the plasticity zone (87). However, even within this region of genetic variation, similarity in putative virulence factors between MoPn and serovar D have been described (8). This high degree of genetic relatedness implies that the strains share common virulence and pathogenic mechanisms and that the antigens recognized by the protective arm of the immune response would be similar or perhaps identical.
Strains of human serovars have been used in the murine genital tract model. Intravaginal inoculation with human serovars typically produces a mild genital tract infection of short duration (77) characterized by low bacterial burdens, minimal submucosal inflammation, and the absence of postinfection sequelae (tubal occlusion, hydrosalpinx, and infertility). In fact, human strains cause postinfection sequelae only following the inoculation of large doses of chlamydiae directly into the uterine horns or ovarian bursa (113, 114). The more invasive LGV strains have also been used in the mouse model. However, infections are only established by intravenous or intraperitoneal inoculation and not following intravaginal challenge. Parenteral inoculation produces infection of reticuloendothelial organs such as the spleen and liver (12), with macrophages, not epithelial cells, as the primary cell target. Thus, immunological findings bearing on mechanisms of host immunity and vaccine efficacy that employ LGV strains could differ significantly from results obtained from infections restricted to the genital mucosae. Although not a full mimic of human infection and disease, infection of the mouse female genital tract with the MoPn strain is presently the best model to study immunity to chlamydial genital tract infection. For these reasons, we will focus our discussion on adaptive immunity to results obtained with the MoPn infection model.
ADAPTIVE IMMUNITY
Three experimental approaches have been used to comprehensively characterize the immunological basis of protective immunity in the murine genital tract infection model, including (i) adoptive transfer of immune lymphocyte populations to naïve mice, (ii) in vivo depletion of specific lymphocyte populations, and (iii) infection of gene knockout mice (Table 1).
TABLE 1.
Summary of in vivo studies used to define immunological parameters important in adaptive immunity to C. trachomatis genital tract infection
| Method | Major deficiencya | Outcome of genital tract infection | Reference(s) |
|---|---|---|---|
| Adoptive transfer | |||
| CD4+ T cells | None | Reduced bacterial shedding; shortened course of infection | 98 |
| CD8+ T cells | None | Minimal effect | 98 |
| In vivo depletion | |||
| Anti-IL-12 | IL-12 | Delayed resolution | 73 |
| Anti-IFN-γ | IFN-γ | Delayed resolution | 84 |
| Anti-IL-4 | IL-4 | No effect | 73 |
| Anti-μ | Antibody | No effect | 82 |
| Anti-CD4 (reinfection) | CD4+ T cells | Reinfection: increased bacterial shedding, infection of longer duration | 64 |
| Anti-CD8 (reinfection) | CD8+ T cells | No effect on secondary reinfection | 64 |
| Anti-CD4 and anti-CD8 (reinfection) | CD4+ and CD8+ T cells | Reinfection: slightly increased bacterial shedding, infection resolves | 63 |
| Anti-CD4 and B-cell deficiency (reinfection) | CD4+ T cells and B cells | Reinfection does not resolve | 64 |
| Anti-CD8 and B-cell deficiency (reinfection) | CD8+ T cells and B cells | No effect on secondary reinfection | 64 |
| Gene knockout miceb | |||
| Nude mice | T-cell immunity | Infection does not resolve | 85 |
| SCID | T- and B-cell immunity | Infection does not resolve | 19 |
| TCRβ chain | αβT cells | Infection does not resolve | 73 |
| Aβ (MHC class II) | MHC class II restricted immunity | Infection does not resolve | 61 |
| IFN-γ | IFN-γ | Delayed resolution and systemic dissemination | 19, 42, 73, 77 |
| IFN-γ receptor | IFN-γ receptor functions | Delayed resolution | 42, 44 |
| CD4 | CD4+ T cells | Delayed resolution | 61 |
| TNFα p55 receptor | TNF-α receptor function | Increased bacterial shedding; infection resolves | 77 |
| ICAM-1 | Leukocyte adhesion | Increased bacterial shedding; infection resolves | 33 |
| μMT | Mature B cells and antibody | No effect on primary infection; increased susceptibility to reinfection | 43, 45, 99 |
| FcRγ/FcγRII | Fc receptor functions | No effect on primary infection; increased bacterial shedding on reinfection | 59 |
| β2M | CD8+-T-cell functions | No effect | 61 |
| TCRγ chain | γδT cells | No effect | 73 |
| Faslpr | CD4+-Th1 and CD8+-T-cell apoptosis | No effect | 75 |
| FasLgld | CD4+-Th1 and CD8+-T-cell apoptosis | No effect | 75 |
| Pfptm (perforin) | CD8+-T-cell cytotoxicity | No effect | 75 |
| PKO/gld (perforin/FasL) | CD4+ Th1 and CD8+ apoptosis and cytotoxicity | No effect | 75 |
| IL-6 | IL-6 | No effect | 74 |
| iNOS | Inducible nitric oxide | No effect | 37, 74, 80 |
| Nramp-1 | Nramp-1 | No effect | 68 |
Major functional immune deficiency.
Abbreviations: ICAM, intracellular adhesion molecule; iNOS, inducible nitric oxide synthase; Nramp, natural resistance-associated macrophage protein; pfp, pore forming protein; PKO, perforin knockout; TCR, T-cell receptor; TNFR, tumor necrosis factor receptor; SCID, severe combined immunodeficiency.
The importance of cell-mediated immunity in immune protection against chlamydial genital tract infection was first demonstrated with T-cell-deficient athymic (nude) mice and mice depleted of CD4+ T cells (54, 85). Subsequently, it was demonstrated that adoptive transfer of polyclonal CD4+ T cells, obtained from postinfection immune mice, confers significant protection to naïve mice, whereas transfer of immune CD8+ T cells does not (98). Chlamydiae-specific CD4+- or CD8+-T-cell lines and clones also impart partial protection to naïve recipient mice; however, the magnitude of protection conferred by CD4+ T cells is markedly superior to that by CD8+ T cells (35, 39, 81, 93). Collectively, many lines of evidence strongly implicate CD4+ T cells as an important lymphocyte subset in mediating antichlamydial immunity.
More recently, chlamydial genital tract infection has been studied with gene knockout mice that have a broad spectrum of well-characterized immunodeficiencies (Table 1). Clearance of primary infection and, when possible, resistance to reinfection have been evaluated in mice with deficiencies in B or T cells, major histocompatibility complex (MHC) class I or class II molecules, T-cell cytokines, molecules that elicit T-cell cytolytic effector functions, and lymphocyte trafficking and adhesion molecules. Importantly, only T-cell receptor αβ and MHC class II deficiencies render mice incapable of resolving genital tract infections (61, 73). Conversely, the resolution of primary infection in β2-microglobulin gene knockout mice (i.e., CD8+-T-cell deficient) and μMT gene knockout mice (i.e., B-cell deficient) is equivalent to that of immunocompetent wild-type mice (43, 45, 61, 99). Hence, neither B cells, antibodies, nor CD8+ T cells contribute an essential effector function for the eradication of primary infection. CD8+-T-cell-deficient mice remain solidly protected following rechallenge (61), whereas B-cell-deficient mice are uniformly susceptible to reinfection and exhibit delayed clearance of chlamydiae (99), implicating a functional role for B cells in adaptive recall immunity. Thus, B cells play an important but undefined effector function in resistance to reinfection. The role of B cells in recall immunity will be addressed in more detail below.
Chlamydial immunity has also been evaluated in mice that are deficient in cytokines, cytokine receptors, or molecules critical to lymphocyte cytolytic effector function. Mice depleted of interleukin-12 (IL-12) or gamma interferon (IFN-γ) or genetically deficient in IFN-γ or the IFN-γ receptor have a marked inability to eradicate primary infection (19, 42, 44, 73, 77, 84). In contrast, tumor necrosis factor alpha (TNF-α) depletion or TNF receptor or IL-6 deficiency has minimal or no measurable effect on the ability of mice to resolve primary infection or resist secondary rechallenge (21, 74, 77). These results clearly demonstrate the importance of T-helper type 1 (Th1) immunity in resistance to chlamydial infection and correlate with other investigations that describe a predominance of Th1 immune responses in the genital tract tissues of chlamydiae-infected mice (15, 73, 76, 77). To investigate the role of cytotoxic T cells in antichlamydial immunity, infection has been studied in mice genetically deficient in perforin, Fas, or Fas ligand or in double-knockout mice deficient in both perforin and Fas ligand (75). Independently or in combination, these deficiencies severely compromise antigen specific CD8+-T-cell-mediated cytotoxicity or apoptosis or CD4+-T-cell-mediated apoptosis (46). Interestingly, mice deficient in cytolytic effector functions clear primary chlamydial infections with kinetics nearly identical to those of wild-type control mice and are resistant to reinfection upon rechallenge (75). These findings, together with the results from CD8-deficient mice described above, present indisputable evidence against an effector function for cytolytic T cells in the eradication and control of chlamydial infection of the murine urogenital epithelium. Conversely, the findings support a key effector function for an IL-12-dependent, CD4+ Th1, IFN-γ-mediated immunity in the clearance of chlamydial genital tract infection.
As noted above, B-cell-deficient mice have an increase in colonization frequency and tend to shed greater numbers of infectious organisms than B-cell-competent mice following rechallenge (99). These findings suggested that B cells play a more critical role in immunity to secondary infection than previously thought and prompted us to conduct experiments that would more clearly disclose the roles of B cells and CD4+ T cells in recall immunity (63, 64). We showed that immune wild-type mice depleted of CD4+ T cells before secondary challenge develop an infection of longer duration and shed greater numbers of bacteria than non-CD4-depleted control mice (Fig. 4A) (64); however, infection resolves by about 3 weeks even in the absence of CD4+ T cells (64). In contrast, immune B-cell-deficient mice depleted of CD4+ T cells before rechallenge are incapable of resolving secondary infection until CD4+ T cells are allowed to repopulate, and infection is characterized by a level of bacterial shedding comparable to that observed in primary infection of naïve animals (Fig. 4B) (64). CD8+ T cells are inconsequential in recall immunity, since the infection that follows secondary rechallenge of CD8-depleted wild-type or CD8-depleted B-cell-deficient mice is not appreciably different from that in nondepleted mice (64). Therefore, both CD4+ T cells and B cells participate importantly in the memory immune response to chlamydial reinfection of the genital tract. It is not known if CD4+ T cells and B cells function independently or cooperatively to generate this important aspect of the host immune response.
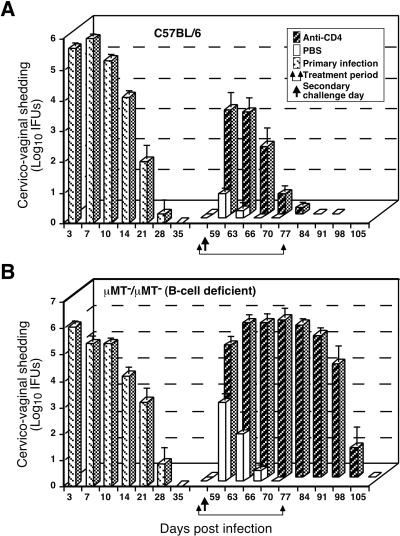
FIG. 4.
Effect of anti-CD4 treatment on the resolution of secondary C. trachomatis MoPn genital tract infection of wild-type C57BL/6 mice (A) and μMT/μMT B-cell-deficient gene knockout mice (B). Mice were infected and IFUs were enumerated as described in the legend to Fig. 1. Following the resolution of primary infection, immune mice were either treated with phosphate-buffered saline (PBS) or anti-CD4 monoclonal antibody and then rechallenged. Mice depleted of CD4+ T cells resolve secondary infection, although resolution is delayed (A), whereas mice depleted of both CD4+ T cells and B cells fail to resolve secondary infection. These data convincingly demonstrate a role for B cells and CD4+ T cells in adaptive immunity to chlamydial genital tract infection. This figure was adapted with permission from reference 64.
EFFECTOR MECHANISMS OF THE ADAPTIVE IMMUNE RESPONSE
CD4+ T cells.
Cellular cytotoxicity and cytokine-mediated functions are two possible effector mechanisms that may participate in the CD4+ Th1 immune response that is absolutely required for host resistance to chlamydial genital tract infection. Cytolysis of infected cells via the Fas-Fas ligand apoptotic pathway is not critical for protective immunity, however, since mice deficient in that pathway resolve genital tract infection like immunocompetent mice (75). Conversely, the Th1 cytokine IFN-γ is essential for optimal clearance of infection from genital tract tissue (19, 42, 44, 73, 77). The effector role for IFN-γ in mediating chlamydial clearance is not known but could include both immunoregulatory and nonregulatory functions. An immunoregulatory function for IFN-γ-producing CD4+ Th1 cells is in the activation of antigen-specific cytotoxic CD8+ T cells. That mechanism seems unlikely, however, because CD8+ T cells are not required for immunity (61, 63, 64, 98). A direct role for IFN-γ in eradicating chlamydiae from the urogenital epithelium therefore seems more likely. In theory, the mechanisms by which IFN-γ could inhibit chlamydial intracellular growth are numerous, due to its pleomorphic effects on host cell function (10). The two IFN-γ-inducible host cell functions that have received the majority of attention in studies of chlamydial immunity are the induction of inducible nitric oxide synthase and of tryptophan-decyclizing enzyme indoleamine 2,3-dioxygenase (14, 34, 40). The production of bactericidal nitric oxide free radicals is not a plausible mechanism because mice genetically deficient in inducible nitric oxide synthase resolve both primary and secondary chlamydial infections with kinetics similar to those of wild-type mice (Table 1) (37, 74, 80). Conversely, the depletion of intracellular tryptophan pools by indoleamine 2,3-dioxygenase is inhibitory to chlamydial growth due to the parasite's tryptophan auxotrophy (1, 6, 7, 14, 30, 48, 60, 90). The MoPn and human strains of C. trachomatis exhibit differences in susceptibility to the growth-inhibiting effects of IFN-γ. Human strains are generally more susceptible to the inhibitory effects of IFN-γ both in vitro and in vivo (60, 77). The biological basis for this difference is not understood but may represent differences in the mechanism(s) by which IFN-γ inhibits intracellular growth of murine and human strains. Regardless of this difference, the sensitivity of human strains to IFN-γ indicates that stimulation of mucosal IFN-γ Th1 responses is a desirable goal for a human antichlamydial vaccine.
B cells and antibodies.
The role of CD4+ T cells and B cells in protective recall immunity to chlamydial genital tract infection is unequivocal (Fig. 4). Understanding the interplay between those cell populations in orchestrating this potent level of adaptive immunity is critically important to vaccine development. How B cells and/or antibodies contribute to adaptive immunity to chlamydial genital tract infection is not understood, but several mechanisms can be proposed that invoke both direct and indirect effector mechanisms. B cells may function independently of cell-mediated immunity by secreting antibodies that neutralize chlamydial infectivity and reduce genital tract colonization by blocking chlamydial attachment to epithelial cells. This protective mechanism is supported by in vitro studies showing that antibodies to the chlamydial major outer membrane protein (MOMP) block attachment, and subsequent infectivity, of chlamydiae to epithelial cells (70, 72, 78, 122, 123). A role for neutralizing antibody in vivo is also suggested by studies demonstrating that mice deficient in antibody are less resistant to reinfection than mice with local (genital tract) antichlamydial antibody (99). The killing of opsonized chlamydiae by professional phagocytic cells is also a potential protective mechanism. However, that mechanism may be more important in preventing dissemination of chlamydiae to distant sites rather than resolving infection of the mucosal epithelium. Antibody might also contribute to the resolution of intracellular infection by an antibody-dependent cellular cytotoxicity (ADCC) mechanism. The potential role for an ADCC mechanism in immunity to chlamydial infection is supported by recent studies using Fc receptor-deficient mice (59). Further support for ADCC comes from studies demonstrating that an immunoglobulin A (IgA)-dependent CD4+-T-cell ADCC mechanism functions in immunity to other intracellular bacterial pathogens such as Salmonella and Shigella (107-110). Several laboratories have also detected chlamydial antigens on the surfaces of infected cells (47, 88, 97, 119) which could function as immune targets for ADCC.
In addition to the direct effector functions of antibody, B cells could also participate in an antibody-independent manner in secondary recall immunity. For example, B cells are important antigen-presenting cells in the recall of memory Th cells and function by promoting the clonal expansion of high frequencies of antigen-specific memory Th cells (55, 115). Therefore, defining the mechanism(s) by which B cells contribute to recall adaptive immunity and the chlamydial antigen(s) recognized by B cells is a challenge of utmost importance. Understanding the role of B cells in adaptive chlamydial immunity will likely be key to the design of a vaccine that is capable of inducing optimal priming and recall of protective antigen-specific CD4+ Th1 responses.
CD8+ cytotoxic T cells.
The obligate intracellular lifestyle of chlamydiae intuitively predicts that antigen-specific MHC-restricted CD8+-T-cell cytolysis would be an important effector function in antichlamydial immunity. However, that premise is not supported by studies utilizing adoptive immunization, in vivo depletion of CD8+ T cells, or targeted gene knockout mice (35, 61, 63, 64, 93, 98). Nevertheless, there is little doubt that CD8+ T cells are induced following infection and there is solid evidence that chlamydiae-specific human and mouse CD8+ T cells are cytotoxic for chlamydiae-infected targets in vitro (5, 27, 50, 51, 53, 86, 92, 93). The explanation for this apparent paradox is not known. However, chlamydiae, like certain viruses (112), have evolved strategies to circumvent recognition by cytotoxic T cells. The molecular basis for this resistance has been recently shown to involve a protease that is secreted by chlamydiae into the infected host cytosol (124-126). The chlamydial protease specifically degrades host transcription factors RX1 and USF-1 that mediate the constitutive and the IFN-γ-inducible expression of MHC class I and class II molecules. This novel strategy may explain, at least in part, how chlamydiae evade the effector functions of cytolytic CD8+ and CD4+ T cells in vivo.
Although cytotoxic CD8+ T cells are inconsequential for protective immunity to chlamydial genital tract infection, some CD8+ T cell clones and lines have been shown to confer a level of protective immunity to naïve mice (35, 93). Protection in those studies, however, is rather modest and mediated by IFN-γ rather than by cellular cytotoxicity (35, 53, 93). Regardless of the mechanism, CD8+ T cells are apparently neither sufficient nor necessary to confer optimum levels of protective immunity in the murine model of chlamydial genital infection.
Some observations suggest that antigen-specific cytotoxic CD8+ T cells may contribute more to the pathogenesis of chlamydial infection than to protective immunity. For example, chlamydiae-specific T-cell-mediated cytolysis requires high lymphocyte-to-target cell ratios and lysis of infected targets occurs late in the chlamydial developmental cycle, at a time when the majority of organisms have differentiated into infectious EBs. That result is not satisfying in terms of a mechanism that would favor inhibition of intracellular growth and argues that cytolytic T cells could potentially contribute more to the pathology than to the eradication of infection. That possibility is supported by the observation that women expressing a particular HLA class I molecule, HLA-A31, are at significantly greater risk of developing chlamydial PID (52), indirectly implicating an immunopathogenetic role for CD8+ T cells. Despite the apparent ability of chlamydiae to interfere with CD8+-T-cell-mediated cytotoxicity, the host has evolved highly effective adaptive immune mechanisms to combat and resist infection. Understanding the mechanisms that contribute to this highly effective adaptive immune response and devising methods to replicate it through vaccination are important goals of future research.
VACCINE PROSPECTIVES AND CHALLENGES
Based on the current understanding of immunity to chlamydial infection of the female murine genital tract and the assumption that results from the mouse model are applicable to human immunity, an immunogen capable of stimulating both protective CD4+ Th1 and B-cell immunity is a highly desirable characteristic of an antichlamydial vaccine. Ideally, the basic requirement of such a vaccine is the induction of long-lived heterotypic immunity that provides coverage against the major C. trachomatis STD serovars (D, E, F, G, H, I, J, and K) and is targeted to the genital tract mucosae. To achieve that goal a more thorough understanding of the effector functions of CD4+ T cells and B cells is needed. In particular, knowledge of the role of B cells in the generation and expansion of antigen-specific Th1 memory immunity and the identification of protective chlamydial antigens that are recognized by both CD4+ T cells and B cells are essential.
To date there has been little progress in the identification of promising candidate vaccine antigens. The most studied antigen is the chlamydial MOMP (omp1). The MOMP is a predominant disulfide cross-linked surface protein and is an immunodominant B-cell antigen (31). MOMP is also the primary serotyping antigen (16, 116). Antibodies specific to MOMP neutralize infectivity by blocking chlamydial attachment to host cells (70, 72, 79, 122, 123), suggesting a role for the protein as a chlamydial adhesin (103, 104). The 40-kDa MOMP is characterized by four symmetrically spaced regions of amino acid variation termed variable domains (VDs) (3, 96). The surface-exposed VDs are the targets of serotyping and neutralizing antibodies (104, 122, 123). The VDs are thought to exist as disulfide-stabilized loops on the surface of the organism that form conformationally important regions critical to the generation of domains that elicit high-affinity neutralizing antibody and mediate host cell interactions (122). The MOMP has been the focus of many vaccination studies because of those important immunological properties and its implication in chlamydial pathogenesis. Recombinant MOMP, MOMP synthetic peptides, DNA vaccines encoding MOMP, and the passive transfer of MOMP-specific monoclonal antibodies have been evaluated for protective efficacy. All studies have yielded disappointing results since protective immunity either was not generated or was partial, at best (18, 25, 32, 36, 67, 70, 71, 79, 102, 120, 121). The reason for the ineffectiveness of MOMP as a vaccine is not known, but it may result from adjuvants or delivery systems that ineffectively target genital tract mucosae or from use of MOMP immunogens that do not mimic the native structure of the protein (69).
Stimulation of mucosal inductive sites in the lungs and the intestine has been proposed to confer an immune response common to many mucosal sites (58). However, such a response has not been clearly established for the genital tract mucosae, a tissue that lacks the organized mucosal lymphoid structures found in the lungs and the gut (65). Both systemic (vascular cellular adhesion molecule) and mucosal (mucosal addresin cellular adhesion molecule) lymphocyte homing molecules are expressed on chlamydiae-infected genital tract tissues (38, 49, 76), but their role in the recruitment and retention of lymphocytes at the genital tract mucosae has not been defined. A better understanding of the immunology of the female genital tract and its relation to the systemic and mucosal immune systems will be key to the development of delivery systems that are capable of specifically targeting MOMP immunity to the genital tract mucosae. The immunogenicity of an effective MOMP vaccine may also depend on our ability to mimic the antigen processing and presentation pathways that occur in the context of a natural infection. Those immunological characteristics are likely key to ensuring an optimum protective recall immune response of appropriate antigen and lymphocyte specificity following natural rechallenge. Thus, the true potential of MOMP as a sole vaccine target may await accomplishment of those achievements.
Clearly, there is a need for studies to identify other potential protective antigens. The genomic sequence of C. trachomatis provides clues for the selection of new antigens, either structural or secreted, that might serve as experimental vaccine targets (87, 94, 95). It is imperative that the evaluation of any vaccine target antigen be conducted in appropriate preclinical models of chlamydial genital tract infection, such as the murine model described herein.
Notable protective immunity against chlamydial genital tract infection has only been achieved through the use of adoptively transferred dendritic cells (DCs) pulsed ex vivo with inactivated chlamydial EBs (100). Immunization with ex vivo-pulsed DCs elicits both chlamydiae-specific antibodies and CD4+ Th1 immune responses, and the level of protection generated is equivalent to that produced by primary infection. This unconventional vaccination approach is not applicable for use in humans, but the results demonstrate that active immunization with nonviable chlamydial organisms is feasible. The potent adjuvant properties and mucosal immunizing capabilities of DCs are likely too complex to be effectively mimicked by traditional vaccine approaches. However, antigen-pulsed DCs are an attractive and potentially very useful system for the delivery and screening of candidate chlamydial subunit or DNA-based vaccines.
Perhaps chlamydial infection of the genital mucosae represents a challenge that is too formidable for traditional vaccines because of the complex biology, antigenic structure, and mucosal immunity requirements of the parasite. Indeed, such complex requirements might be best met by attenuated chlamydial strains that colonize and infect epithelial cells of the urogenital tract (13, 101). The potential usefulness of this approach has been demonstrated using temperature-sensitive mutants of C. psittaci for vaccination against ovine abortion (17, 89). Major obstacles in generating and isolating attenuated C. trachomatis strains are the inability to genetically manipulate the organism and to isolate and propagate clonal lineages. Plaque cloning techniques have recently been developed and will facilitate the isolation of clonal lineages of chlamydiae (56). However, genetic transformation systems for use with chlamydiae have not been developed. Advances in chlamydial genomics may provide the information necessary for the development of a chlamydial transformation system and may facilitate the generation and selection of attenuated chlamydial strains.
CONCLUSIONS
Chlamydial urogenital infections remain a public health problem. It has been proposed that the development of a vaccine to protect against chlamydial infection would have a profound impact on preventing the serious sequelae of chlamydial infections and reducing healthcare costs worldwide. We have discussed protective immunity as it develops in the mouse following chlamydial genital tract infection and why the mouse is an excellent model in which to study adaptive immunity to this intracellular pathogen and to test experimental vaccines. Recently, Brunham reviewed human immunity to chlamydial infection (11). Apparent from that collection of literature are the parallels in disease processes and immune responses that develop following chlamydial infection of the mouse and human. Common to murine and human chlamydial genital tract infections are (i) the development of protective immunity, (ii) the histopathology, (iii) the importance of Th1 CD4+ T cells in immunity, (iv) the induction of chlamydiae-reactive CD8+ T cells following infection, but doubts about their role in vivo, and (v) the noted but as yet undefined role of B cells and antibody in protective immunity. These similarities in chlamydial pathogenesis and immunity strongly support the use of the murine model for immunity and vaccine studies. Successful vaccine development, however, will depend on research that extends the findings from the murine model to that of primate and human genital tract infection.
Acknowledgments
We thank J. M. Musser, K. Hasenkrug, K. Peterson, and S. G. Morrison for their critical review and editorial assistance with the manuscript. We are grateful to G. Hetrick for assistance with graphics.
R.P.M. is supported by a grant from the National Institutes of Health (AI-38991).
Editor: D. A. Portnoy
REFERENCES
- 1.Allan, I., and J. H. Pearce. 1983. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J. Gen. Microbiol. 129:2001-2007. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, T., P. Saikku, P. Koskela, A. Bloigu, J. Dillner, I. Ikaheimo, E. Jellum, M. Lehtinnen, P. Lenner, T. Hakulinen, A. Narvanen, E. Pukkala, S. Thoresen, L. Youngman, and J. Paavonen. 2001. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA 285:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Baehr, W., Y.-X. Zhang, T. Joseph, H. Su, F. E. Nano, K. D. E. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143: 63-66. [DOI] [PubMed] [Google Scholar]
- 5.Beatty, P. R., and R. S. Stephens. 1994. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J. Immunol. 153:4588-4595. [PubMed] [Google Scholar]
- 6.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon-gamma mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belland, R. J., M. A. Scidmore, D. D. Crane, D. M. Hogan, W. Whitmire, G. McClarty, and H. D. Caldwell. 2001. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc. Natl. Acad. Sci. USA 98:13984-13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, C. M. 1997. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin. Microbiol. Rev. 10:160-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 11.Brunham, R. C. 1999. Human immunity to chlamydiae, p. 211-238. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 12.Brunham, R. C., C.-C. Kuo, and W.-J. Chen. 1985. Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infect. Immun. 48:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunham, R. C., D. J. Zhang, X. Yang, and G. McClarty. 2000. The potential for vaccine development against chlamydial infection and disease. J. Infect. Dis. 181 (Suppl. 3):S538-S543. [DOI] [PubMed]
- 14.Byrne, G. I., L. K. Lehmann, and G. J. Landry. 1986. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell, H. D., and J. Schachter. 1982. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 35:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalmers, W. S., J. Simpson, S. J. Lee, and W. Baxendale. 1997. Use of a live chlamydial vaccine to prevent ovine enzootic abortion. Vet. Rec. 141:63-67. [DOI] [PubMed] [Google Scholar]
- 18.Cotter, T. W., Q. Meng, Z.-L. Shen, Y.-X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darville, T., C. W. Andrews, Jr., K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darville, T., C. W. Andrews, Jr., and R. G. Rank. 2000. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect. Immun. 68:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, and R. G. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 69:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Maza, M. A., and L. M. de la Maza. 1995. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine 13:119-127. [DOI] [PubMed] [Google Scholar]
- 25.Dong-Ji, Z., X. Yang, C. Shen, H. Lu, A. Murdin, and R. C. Brunham. 2000. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect. Immun. 68:3074-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, K. D., R. M. Bush, and A. A. Anderson. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 27.Fling, S. P., R. A. Sutherland, L. N. Steele, B. Hess, S. E. F. D'Orazio, J.-F. Maisonneuve, M. F. Lampe, P. Probst, and M. N. Starnbach. 2001. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 98:1160-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grayston, J. T. 2000. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J. Infect. Dis. 181:S402-S410. [DOI] [PubMed]
- 29.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 30.Hanna, L., T. C. Merigan, and E. Jawetz. 1966. Inhibition of TRIC agents by virus-induced interferon. Proc. Soc. Exp. Biol. Med. 122:417-421. [DOI] [PubMed] [Google Scholar]
- 31.Hatch, T. P. 1999. Developmental biology, p. 29-67. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 32.Hayes, L. J., J. W. Conlan, J. S. Everson, M. E. Ward, and I. N. Clarke. 1991. Chlamydia trachomatis major outer membrane protein epitopes expressed as fusions with LamB in an attenuated aroA strain of Salmonella typhimurium; their application as potential immunogens. J. Gen. Microbiol. 137:1557-1564. [DOI] [PubMed] [Google Scholar]
- 33.Igietseme, J. U., G. A. Ananaba, J. Bolier, S. Bowers, T. Moore, T. Belay, D. Lyn, and C. M. Black. 1999. The intracellular adhesion molecule type-1 is required for rapid activation of T helper type 1 lymphocytes that control early acute phase of genital chlamydial infection in mice. Immunology 98:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igietseme, J. U., G. A. Ananaba, D. H. Candal, D. Lyn, and C. M. Black. 1998. Immune control of chlamydial growth in the human epithelial cell line RT4 involves multiple mechanisms that induce nitric oxide induction, tryptophan catabolism and iron deprivation. Microbiol. Immunol. 42:617-625. [DOI] [PubMed] [Google Scholar]
- 35.Igietseme, J. U., D. M. Magee, D. M. Williams, and R. G. Rank. 1994. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect. Immun. 62:5195-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igietseme, J. U., and A. Murdin. 2000. Induction of protective immunity against Chlamydia trachomatis genital infection by a vaccine based on major outer membrane protein-lipophilic immune response-stimulating complexes. Infect. Immun. 68:6798-6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igietseme, J. U., L. L. Perry, G. A. Ananaba, I. M. Uriri, O. O. Ojior, S. H. Kumar, and H. D. Caldwell. 1998. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect. Immun. 66:1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igietseme, J. U., J. L. Portis, and L. L. Perry. 2001. Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect. Immun. 69:1832-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 40.Igietseme, J. U., I. M. Uriri, R. Hawkins, and R. G. Rank. 1996. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J. Leukoc. Biol. 59:656-662. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine. Committee on Prevention and Control of Sexually Transmitted Diseases. 1997. The hidden epidemic: confronting sexually transmitted diseases. National Academy Press, Washington, D.C.
- 42.Ito, J. I., and J. M. Lyons. 1999. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect. Immun. 67:5518-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson, M., and N. Lycke. 2001. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology 102:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 65:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson, M., M. Ward, and N. Lycke. 1997. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology 92:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-232. [DOI] [PubMed] [Google Scholar]
- 47.Karimi, S. T., R. H. Schloemer, and C. E. Wilde III. 1989. Accumulation of chlamydial lipopolysaccharide antigen in the plasma membranes of infected cells. Infect. Immun. 57:1780-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kazar, J., J. D. Gillmore, and F. B. Gordon. 1971. Effect of interferon and interferon inducers on infections with a nonviral intracellular microorganism, Chlamydia trachomatis. Infect. Immun. 3:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly, K. A., and R. G. Rank. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, S.-K., K. Angevine, K. Demick, L. Ortiz, R. Rudersdorf, D. Watkins, and R. DeMars. 1999. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J. Immunol. 162:6855-6866. [PubMed] [Google Scholar]
- 51.Kim, S.-K., L. Devine, M. Angevine, R. DeMars, and P. B. Kavathas. 2000. Direct detection and magnetic isolation of Chlamydia trachomatis major outer membrane protein-specific CD8+ CTLs with HLA class I tetramers. J. Immunol. 165:7285-7299. [DOI] [PubMed] [Google Scholar]
- 52.Kimani, J., I. W. Maclean, J. J. Bwayo, K. MacDonald, J. Oyugi, G. M. Maitha, R. W. Peeling, M. Cheang, N. J. D. Nagelkerke, F. A. Plummer, and R. C. Brunham. 1996. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J. Infect. Dis. 173: 1437-1444. [DOI] [PubMed] [Google Scholar]
- 53.Lampe, M. F., C. B. Wilson, M. J. Bevan, and M. N. Starnbach. 1998. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect. Immun. 66:5457-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landers, D. V., K. Erlich, M. Sung, and J. Schachter. 1991. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect. Immun. 59:3774-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linton, P.-J., J. Harbertson, and L. M. Bradley. 2000. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 165:5558-5565. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto, A., H. Izutsu, N. Miyashita, and M. Ohuchi. 1998. Plaque formation by and plaque cloning of Chlamydia trachomatis biovar trachoma. J. Clin. Microbiol. 36:3013-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClarty, G. 1999. Chlamydial metabolism as inferred from the complete genome sequence, p. 69-100. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 58.McGhee, J. R., J. Mestecky, M. T. Dertzbaugh, J. H. Eldridge, M. Hirasawa, and H. Kiyono. 1992. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10:75-88. [DOI] [PubMed] [Google Scholar]
- 59.Moore, T., G. A. Ananaba, J. Bolier, S. Bowers, T. Belay, F. O. Eko, and J. U. Igietseme. 2002. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology 105:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison, R. P. 2000. Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infect. Immun. 68:6038-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison, S. G., and R. P. Morrison. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 68:2870-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandi, D., and J. P. Allison. 1993. Characterization of neutrophils and T lymphocytes associated with the murine vaginal epithelium. Reg. Immunol. 5:332-338. [PubMed] [Google Scholar]
- 66.Ohshige, K., S. Morio, S. Mizushima, K. Kitamura, K. Tajima, A. Suyama, S. Usuku, P. Tia, L. B. Hor, S. Heng, V. Saphonn, O. Tochikubo, and K. Soda. 2000. Behavioral and serological human immunodeficiency virus risk factors among female commercial sex workers in Cambodia. Int. J. Epidemiol. 29: 344-354. [DOI] [PubMed] [Google Scholar]
- 67.Pal, S., K. M. Barnhart, Q. Wei, A. M. Abai, E. M. Peterson, and L. M. de la Maza. 1999. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine 17:459-465. [DOI] [PubMed] [Google Scholar]
- 68.Pal, S., E. M. Peterson, and L. M. de la Maza. 2000. Role of Nramp1 deletion in Chlamydia infection in mice. Infect. Immun. 68:4831-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pal, S., I. Theodor, E. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pal, S., I. Theodor, E. M. Peterson, and L. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 71.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 65:3361-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peeling, R., I. W. Maclean, and R. C. Brunham. 1984. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect. Immun. 46:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 74.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1998. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect. Immun. 66:1265-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perry, L. L., K. Feilzer, S. Hughes, and H. D. Caldwell. 1999. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infect. Immun. 67:1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry, L. L., K. Feilzer, J. L. Portis, and H. D. Caldwell. 1998. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J. Immunol. 160:2905-2914. [PubMed] [Google Scholar]
- 77.Perry, L. L., H. Su, K. Feilzer, R. Messer, S. Hughes, W. Whitmire, and H. D. Caldwell. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-γ-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 78.Peterson, E. M., X. Cheng, B. A. Markoff, T. J. Fielder, and L. M. de la Maza. 1991. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect. Immun. 59:4147-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peterson, E. M., X. Cheng, V. L. Motin, and L. M. de la Maza. 1997. Effect of immunoglobulin G isotype on the infectivity of Chlamydia trachomatis in a mouse model of intravaginal infection. Infect. Immun. 65:2693-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramsey, K. H., G. S. Miranpuri, C. E. Poulsen, N. B. Marthakis, L. M. Braune, and G. I. Byrne. 1998. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect. Immun. 66:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramsey, K. H., and R. G. Rank. 1991. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 59:925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramsey, K. H., L. S. F. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rank, R. G. 1999. Models of immunity, p. 239-295. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 84.Rank, R. G., K. H. Ramsey, E. A. Pack, and D. M. Williams. 1992. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect. Immun. 60:4427-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rank, R. G., L. S. F. Soderberg, and A. L. Barron. 1985. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect. Immun. 48:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rasmussen, S. J., P. Timms, P. R. Beatty, and R. S. Stephens. 1996. Cytotoxic-T-lymphocyte-mediated cytolysis of L cells persistently infected with Chlamydia spp. Infect. Immun. 64:1944-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richmond, S. J., and P. Stirling. 1981. Localization of chlamydial group antigen in McCoy cell monolayers infected with Chlamydia trachomatis or Chlamydia psittaci. Infect. Immun. 34:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodolakis, A., and F. Bernard. 1984. Vaccination with temperature-sensitive mutant of Chlamydia psittaci against enzootic abortion of ewes. Vet. Rec. 114:193-194. [DOI] [PubMed] [Google Scholar]
- 90.Rothermel, C. D., G. I. Byrne, and E. A. Havell. 1983. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect. Immun. 39:362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 92.Starnbach, M. N., M. J. Bevan, and M. F. Lampe. 1995. Murine cytotoxic T lymphocytes induced following Chlamydia trachomatis intraperitoneal or genital tract infection respond to cells infected with multiple serovars. Infect. Immun. 63:3527-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starnbach, M. N., M. J. Bevan, and M. F. Lampe. 1994. Protective cytotoxic T-lymphocytes are induced during murine infection with Chlamydia trachomatis. J. Immunol. 153:5183-5189. [PubMed] [Google Scholar]
- 94.Stephens, R. S. 1999. Genomic autobiographies of chlamydiae, p. 9-27. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 95.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 96.Stephens, R. S., G. Mullenbach, R. Sanchez-Pescador, and N. Agabian. 1986. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J. Bacteriol. 168:1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stuart, E. S., P. B. Wyrick, J. Choong, S. B. Stoler, and A. B. MacDonald. 1991. Examination of chlamydial glycolipid with monoclonal antibodies: cellular distribution and epitope binding. Immunology 74:740-747. [PMC free article] [PubMed] [Google Scholar]
- 98.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su, H., R. Messer, W. Whitmire, S. Hughes, and H. D. Caldwell. 2000. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for the development of live attenuated chlamydial vaccine strains. Infect. Immun. 68:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su, H., M. Parnell, and H. D. Caldwell. 1995. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine 13:1023-1032. [DOI] [PubMed] [Google Scholar]
- 103.Su, H., N. G. Watkins, Y.-X. Zhang, and H. D. Caldwell. 1990. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect. Immun. 58:1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su, H., Y.-X. Zhang, O. Barrera, N. G. Watkins, and H. D. Caldwell. 1988. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect. Immun. 56:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Swenson, C. E., E. Donegan, and J. Schachter. 1983. Chlamydia trachomatis-induced salpingitis in mice. J. Infect. Dis. 148: 1101-1107. [DOI] [PubMed] [Google Scholar]
- 106.Swenson, C. E., and J. Schachter. 1984. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex. Transm. Dis. 11:64-67. [DOI] [PubMed] [Google Scholar]
- 107.Tagliabue, A., D. Boraschi, L. Villa, D. F. Keren, G. H. Lowell, R. Rappuoli, and L. Nencioni. 1984. IgA-dependent cell-mediated activity against enteropathogenic bacteria: distribution, specificity, and characterization of the effector cells. J. Immunol. 133:988-992. [PubMed] [Google Scholar]
- 108.Tagliabue, A., L. Nencioni, L. Villa, D. F. Keren, G. H. Lowell, and D. Boraschi. 1983. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature 306: 184-186. [DOI] [PubMed] [Google Scholar]
- 109.Tagliabue, A., L. Villa, D. Boraschi, G. Peri, V. De Gori, and L. Nencioni. 1985. Natural anti-bacterial activity against Salmonella typhi by human T4+ lymphocytes armed with IgA antibodies. J. Immunol. 135:4178-4182. [PubMed] [Google Scholar]
- 110.Tagliabue, A., L. Villa, M. T. De Magistris, M. Romano, S. Silvestri, D. Boraschi, and L. Nencioni. 1986. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J. Immunol. 137:1504-1510. [PubMed] [Google Scholar]
- 111.Thylefors, B., A. D. Negrel, R. Pararajasegaram, and K. Y. Dadzie. 1995. Global data on blindness. Bull. W. H. O. 73:115-121. [PMC free article] [PubMed] [Google Scholar]
- 112.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 113.Tuffrey, M., F. Alexander, and D. Taylor-Robinson. 1990. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J. Exp. Pathol. 71:403-410. [PMC free article] [PubMed] [Google Scholar]
- 114.Tuffrey, M., P. Falder, J. Gale, and D. Taylor-Robinson. 1986. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br. J. Exp. Pathol. 67:605-616. [PMC free article] [PubMed] [Google Scholar]
- 115.van Essen, D., P. Dullforce, T. Brocker, and D. Gray. 2000. Cellular interactions involved in Th cell memory. J. Immunol. 165:3640-3646. [DOI] [PubMed] [Google Scholar]
- 116.Wang, S.-P., C.-C. Kuo, R. C. Barnes, R. S. Stephens, and J. T. Grayston. 1985. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J. Infect. Dis. 152: 791-800. [DOI] [PubMed] [Google Scholar]
- 117.Ward, M. E. 1999. Mechanisms of Chlamydia-induced disease, p. 171-210. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 118.World Health Organization. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. World Health Organization, Geneva, Switzerland.
- 119.Wyrick, P. B., J. Choong, S. T. Knight, D. Goyeau, E. S. Stuart, and A. B. MacDonald. 1994. Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immunol. Infect. Dis. 4:131-141. [Google Scholar]
- 120.Zhang, D.-J., X. Yang, J. Berry, C. Shen, G. McClarty, and R. C. Brunham. 1997. DNA vaccination with the major outer membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J. Infect. Dis. 176: 1035-1040. [DOI] [PubMed] [Google Scholar]
- 121.Zhang, D. J., X. Yang, C. Shen, and R. C. Brunham. 1999. Characterization of immune responses following intramuscular DNA immunization with the MOMP gene of Chlamydia trachomatis mouse pneumonitis strain. Immunology 96:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang, Y.-X., S. Stewart, T. Joseph, H. R. Taylor, and H. D. Caldwell. 1987. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J. Immunol. 138:575-581. [PubMed] [Google Scholar]
- 123.Zhang, Y.-X., S. J. Stewart, and H. D. Caldwell. 1989. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 57:636-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhong, G., T. Fan, and L. Liu. 1999. Chlamydia inhibits interferon g-inducible major histocompatibility complex II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 189:1931-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong, G., L. Liu, T. Fan, P. Fan, and H. Ji. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon-γ-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J. Exp. Med. 191:1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]