Abstract
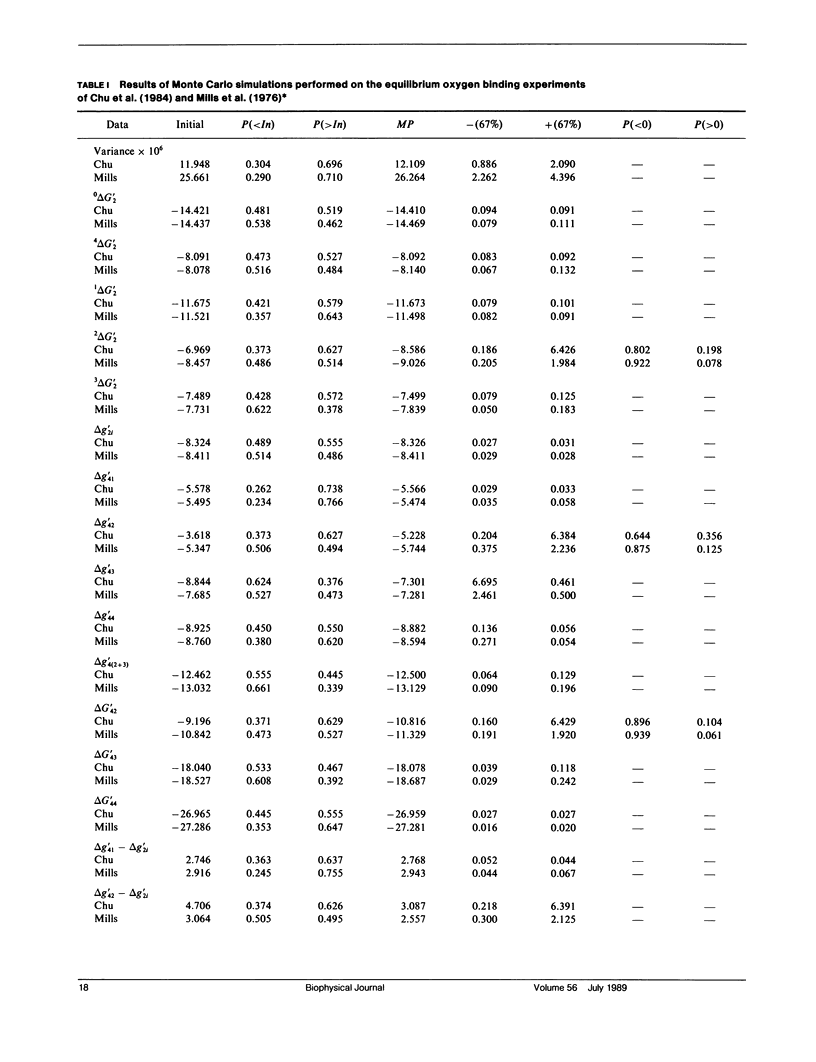
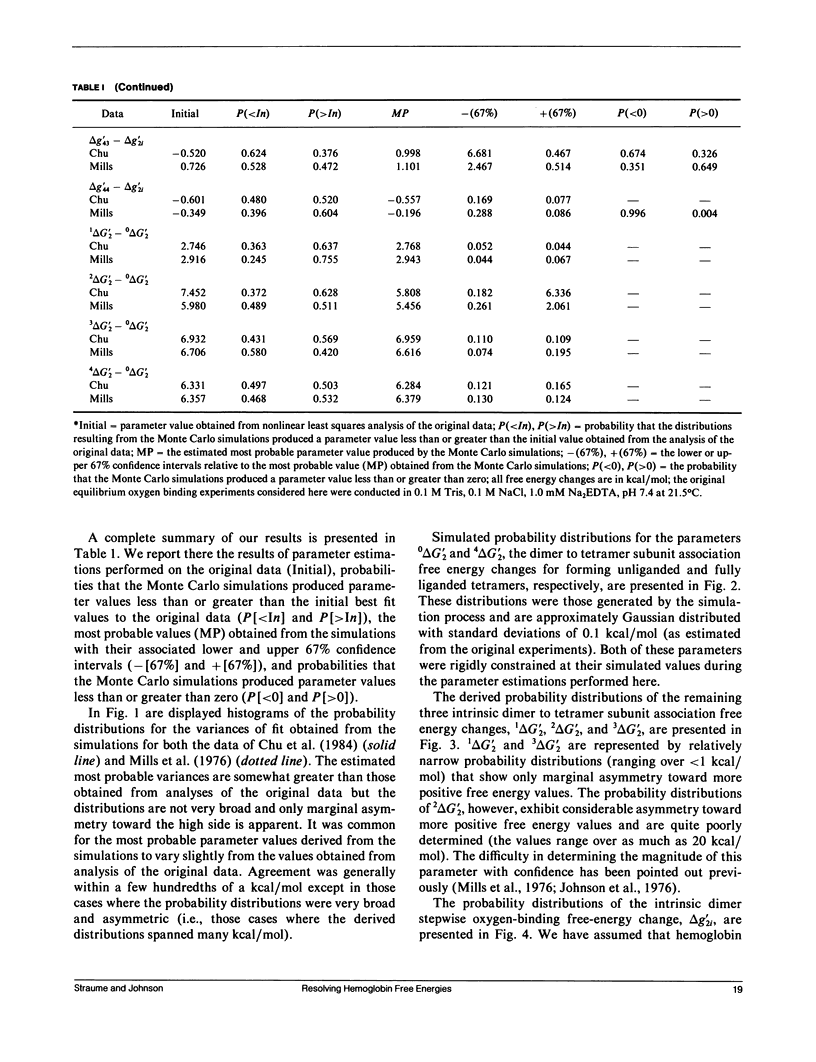
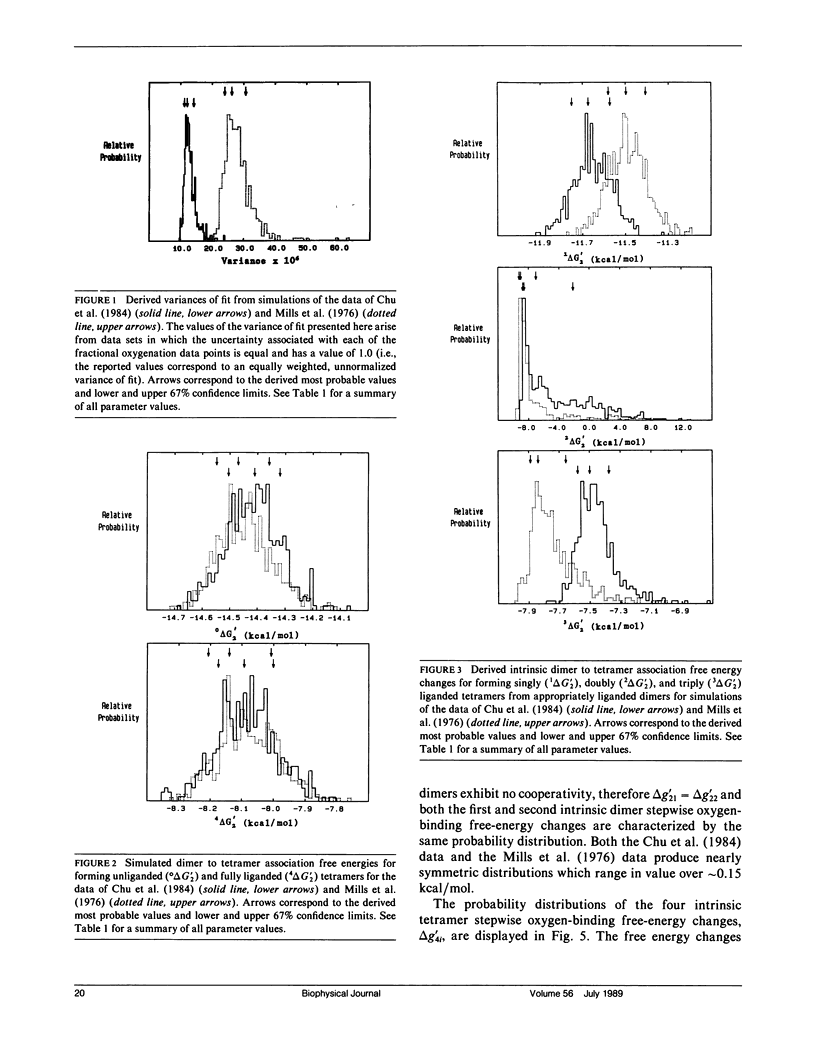
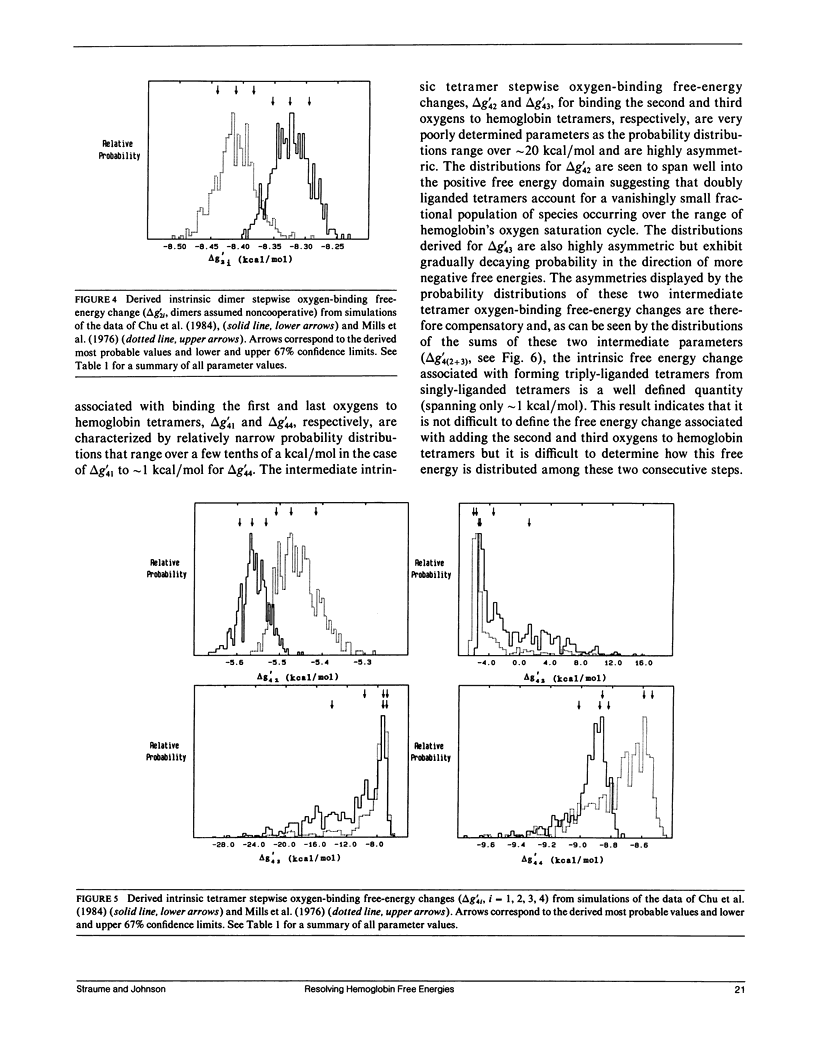
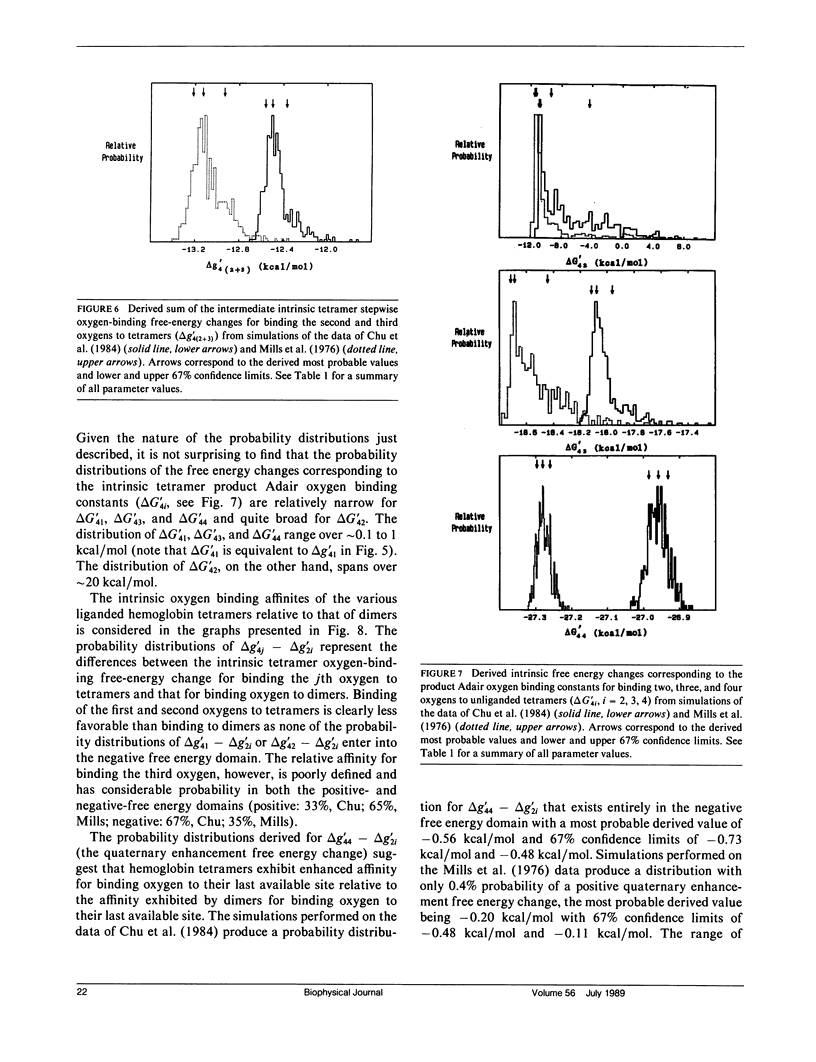
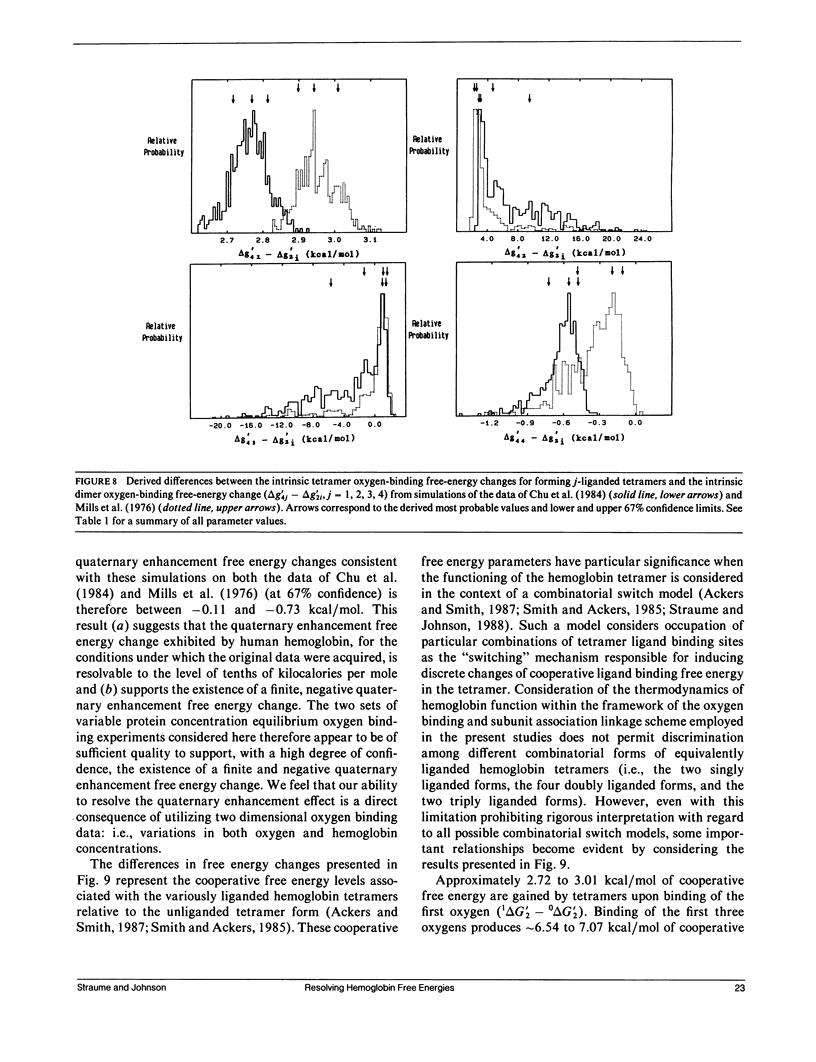
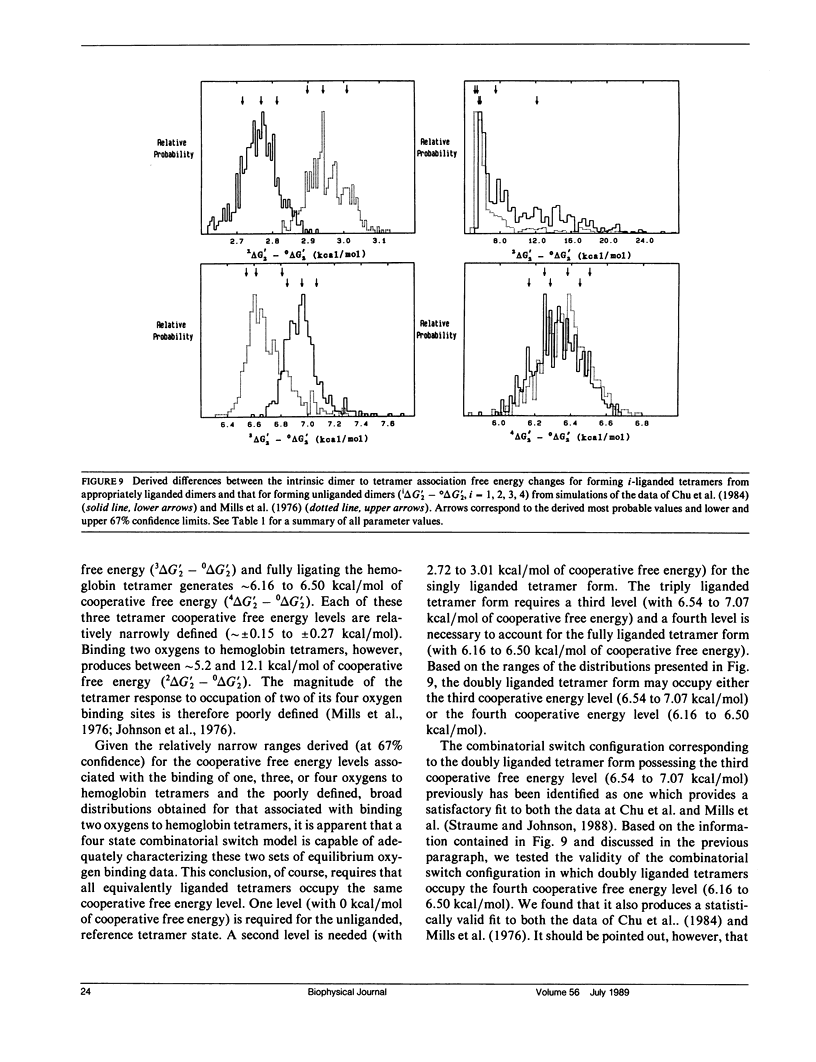
Probability distributions of the free energy changes for oxygen binding, subunit association, and quaternary enhancement by human hemoglobin were obtained from Monte Carlo simulations performed on two independent sets of variable protein concentration equilibrium oxygen-binding data. Uncertainties in unliganded and fully liganded dimer to tetramer association free energy changes (0 delta G'2 and 4 delta G'2) were accounted for in the simulations. Distributions of the dimer to tetramer association free energy changes for forming singly and triply liganded tetramers (1 delta G'2 and 3 delta G'2) are well defined and quite symmetric, whereas that for forming doubly liganded tetramers (2 delta G'2) is poorly defined and highly asymmetric. The distribution of the dimer stepwise oxygen-binding free-energy change (delta g'2i) is well defined and quite symmetric as are those of the tetramer stepwise oxygen-binding free-energy changes for binding the first and last oxygens to tetramers (delta g'41 and delta g'44). Distributions of the intermediate tetramer stepwise oxygen-binding free-energy changes (delta g'42 and delta g'43) are poorly defined and highly asymmetric, but are compensatory in that their sum (delta g'4[2 + 3]) is again well defined and nearly symmetric. Distributions of the free energy changes corresponding to the tetramer product Adair oxygen binding constants (delta G'4i) are well defined and quite symmetric for i = 1, 3, 4 but not for i = 2. The distribution of delta g'44 - delta g'2i (the quaternary enhancement free energy change) is relatively narrow, nearly symmetric, and confined to the negative free-energy domain. This suggests that the quaternary enhancement free energy change (a) may be resolved with good confidence from this data and (b) is finite and negative under the conditions of these experiments. Our results also suggest two different four-state combinatorial switch models that provide accurate characterization of hemoglobin's functional behavior.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Halvorson H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4312–4316. doi: 10.1073/pnas.71.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackers G. K., Smith F. R. The hemoglobin tetramer: a three-state molecular switch for control of ligand affinity. Annu Rev Biophys Biophys Chem. 1987;16:583–609. doi: 10.1146/annurev.bb.16.060187.003055. [DOI] [PubMed] [Google Scholar]
- Doyle M. L., Di Cera E., Gill S. J. Effect of differences in optical properties of intermediate oxygenated species of hemoglobin A0 on Adair constant determination. Biochemistry. 1988 Jan 26;27(2):820–824. doi: 10.1021/bi00402a049. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H., Edelstein S. J. Oxygen binding and subunit interaction of hemoglobin in relation to the two-state model. J Biol Chem. 1987 Jan 15;262(2):516–519. [PubMed] [Google Scholar]
- Gill S. J., Di Cera E., Doyle M. L., Bishop G. A., Robert C. H. Oxygen binding constants for human hemoglobin tetramers. Biochemistry. 1987 Jun 30;26(13):3995–4002. doi: 10.1021/bi00387a038. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Halvorson H. R., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: analysis of linkage functions for constituent energy terms. Biochemistry. 1976 Nov 30;15(24):5363–5371. doi: 10.1021/bi00669a024. [DOI] [PubMed] [Google Scholar]
- Mills F. C., Johnson M. L., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: experimental studies on the concentration dependence of oxygenation curves. Biochemistry. 1976 Nov 30;15(24):5350–5362. doi: 10.1021/bi00669a023. [DOI] [PubMed] [Google Scholar]
- Smith F. R., Ackers G. K. Experimental resolution of cooperative free energies for the ten ligation states of human hemoglobin. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5347–5351. doi: 10.1073/pnas.82.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume M., Johnson M. L. Three-state combinatorial switch models as applied to the binding of oxygen by human hemoglobin. Biochemistry. 1988 Feb 23;27(4):1302–1310. doi: 10.1021/bi00404a032. [DOI] [PubMed] [Google Scholar]


