Abstract
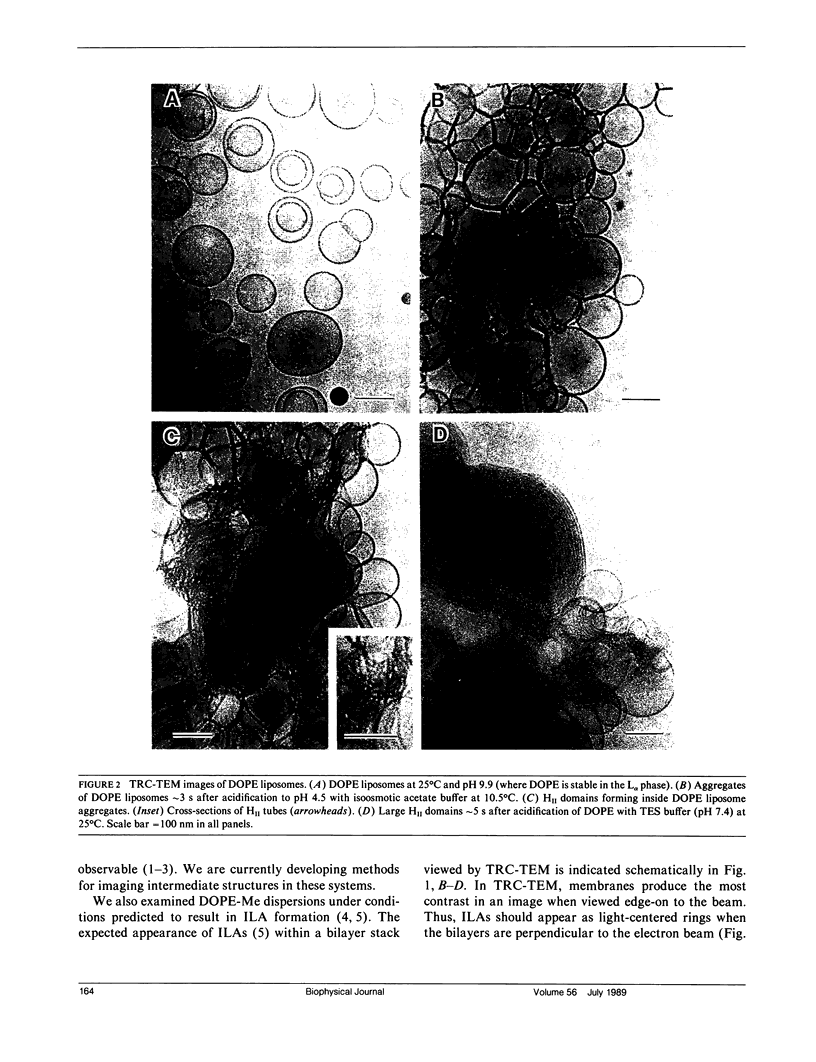
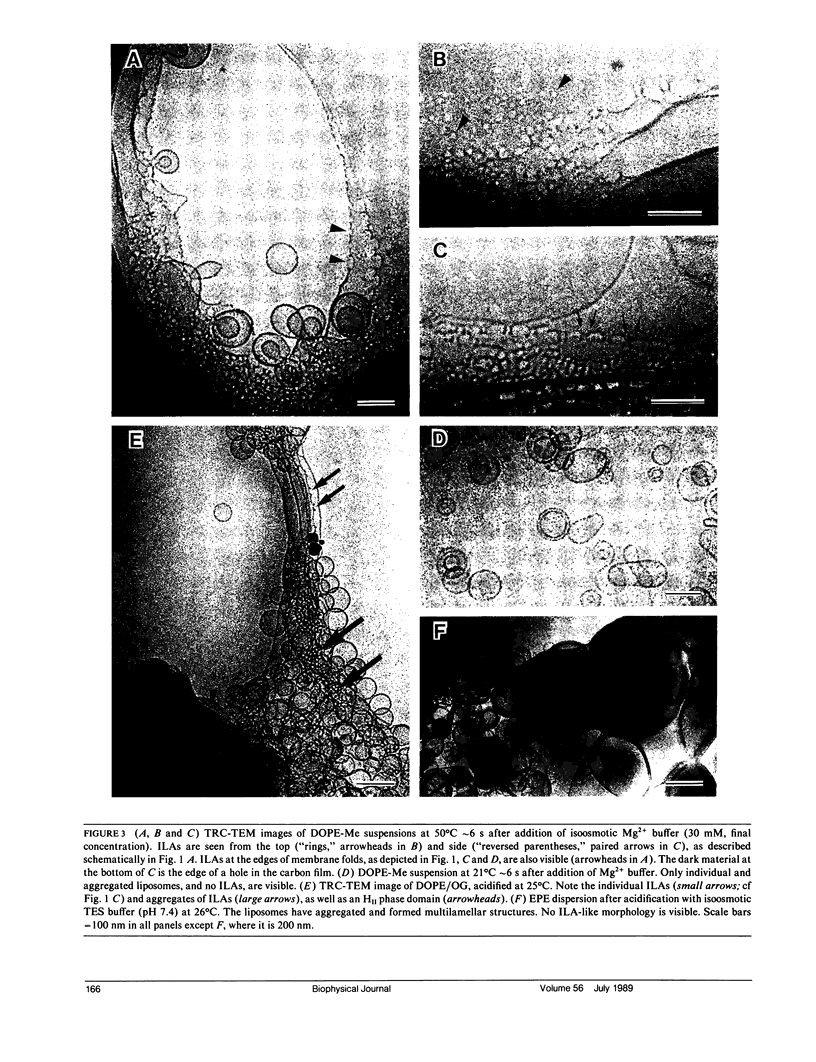
Bilayer-to-nonbilayer phase transitions in phospholipids occur by means of poorly characterized intermediates. Many have proposed that membrane fusion can also occur by formation of these intermediates. Structures for such intermediates were proposed in a recent theory of these transition mechanisms. Using time-resolved cryo-transmission electron Microscopy (TRC-TEM), we have directly visualized the evolution of inverted phase micro-structure in liposomal aggregates. We have identified one of the proposed intermediates, termed an interlamellar attachment (ILA), which has the structure and dimensions predicted by the theory. We show that ILAs are likely to be the structure corresponding to "lipidic particles" observed by freeze-fracture electron microscopy. ILAs appear to assemble the inverted cubic (III) phase by formation of an ILA lattice, as previously proposed. ILAs are also observed to mediate membrane fusion in the same systems, on the same time scale, and under nearly the same conditions in which membrane fusion was observed by fluorescence methods in earlier studies. These earlier studies indicated a linkage between a membrane fusion mechanism and III phase formation. Our micrographs suggest that the same intermediate structure mediates both of those processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Barbet J., Machy P., Truneh A., Leserman L. D. Weak acid-induced release of liposome-encapsulated carboxyfluorescein. Biochim Biophys Acta. 1984 May 30;772(3):347–356. doi: 10.1016/0005-2736(84)90152-4. [DOI] [PubMed] [Google Scholar]
- Bellare J. R., Davis H. T., Scriven L. E., Talmon Y. Controlled environment vitrification system: an improved sample preparation technique. J Electron Microsc Tech. 1988 Sep;10(1):87–111. doi: 10.1002/jemt.1060100111. [DOI] [PubMed] [Google Scholar]
- Bentz J., Ellens H., Szoka F. C. Destabilization of phosphatidylethanolamine-containing liposomes: hexagonal phase and asymmetric membranes. Biochemistry. 1987 Apr 21;26(8):2105–2116. doi: 10.1021/bi00382a008. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim Biophys Acta. 1978 Oct 19;513(1):31–42. doi: 10.1016/0005-2736(78)90109-8. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. Destabilization of phosphatidylethanolamine liposomes at the hexagonal phase transition temperature. Biochemistry. 1986 Jan 28;25(2):285–294. doi: 10.1021/bi00350a001. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. Fusion of phosphatidylethanolamine-containing liposomes and mechanism of the L alpha-HII phase transition. Biochemistry. 1986 Jul 15;25(14):4141–4147. doi: 10.1021/bi00362a023. [DOI] [PubMed] [Google Scholar]
- Ellens H., Siegel D. P., Alford D., Yeagle P. L., Boni L., Lis L. J., Quinn P. J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989 May 2;28(9):3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Frederik P. M., Stuart M. C., Verkleij A. J. Intermediary structures during membrane fusion as observed by cryo-electron microscopy. Biochim Biophys Acta. 1989 Feb 27;979(2):275–278. doi: 10.1016/0005-2736(89)90445-8. [DOI] [PubMed] [Google Scholar]
- Gagné J., Stamatatos L., Diacovo T., Hui S. W., Yeagle P. L., Silvius J. R. Physical properties and surface interactions of bilayer membranes containing N-methylated phosphatidylethanolamines. Biochemistry. 1985 Jul 30;24(16):4400–4408. doi: 10.1021/bi00337a022. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Boni L. T. Lipidic particles and cubic phase lipids. Nature. 1982 Mar 11;296(5853):175–176. doi: 10.1038/296175a0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P. 'Lipidic particles' are intermembrane attachment sites. Nature. 1981 Apr 2;290(5805):427–428. doi: 10.1038/290427a0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Boni L. T. The nature of lipidic particles and their roles in polymorphic transitions. Chem Phys Lipids. 1983 Aug;33(2):113–126. doi: 10.1016/0009-3084(83)90015-4. [DOI] [PubMed] [Google Scholar]
- Miller R. G. Do 'lipidic particles' represent intermembrane attachment sites? Nature. 1980 Sep 11;287(5778):166–167. doi: 10.1038/287166a0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Reese T. S., Miller R. G. Phospholipid bilayer deformations associated with interbilayer contact and fusion. Nature. 1981 Sep 17;293(5829):237–238. doi: 10.1038/293237a0. [DOI] [PubMed] [Google Scholar]
- Sen A., Williams W. P., Brain A. P., Dickens M. J., Quinn P. J. Formation of inverted micelles in dispersions of mixed galactolipids. Nature. 1981 Oct 8;293(5832):488–490. doi: 10.1038/293488a0. [DOI] [PubMed] [Google Scholar]
- Shyamsunder E., Gruner S. M., Tate M. W., Turner D. C., So P. T., Tilcock C. P. Observation of inverted cubic phase in hydrated dioleoylphosphatidylethanolamine membranes. Biochemistry. 1988 Apr 5;27(7):2332–2336. doi: 10.1021/bi00407a014. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal amphiphile phases. III. Isotropic and inverted cubic state formation via intermediates in transitions between L alpha and HII phases. Chem Phys Lipids. 1986 Dec 31;42(4):279–301. doi: 10.1016/0009-3084(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. I. Mechanism of the L alpha----HII phase transitions. Biophys J. 1986 Jun;49(6):1155–1170. doi: 10.1016/S0006-3495(86)83744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986 Jun;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984 Feb;45(2):399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Mombers C., Gerritsen W. J., Leunissen-Bijvelt L., Cullis P. R. Fusion of phospholipid vesicles in association with the appearance of lipidic particles as visualized by freeze fracturing. Biochim Biophys Acta. 1979 Aug 7;555(2):358–361. doi: 10.1016/0005-2736(79)90175-5. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Momvers C., Leunissen-Bijvelt J., Ververgaert P. H. Lipidic intramembranous particles. Nature. 1979 May 10;279(5709):162–163. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., van Echteld C. J., Gerritsen W. J., Cullis P. R., de Kruijff B. The lipidic particle as an intermediate structure in membrane fusion processes and bilayer to hexagonal HII transitions. Biochim Biophys Acta. 1980 Aug 14;600(3):620–624. doi: 10.1016/0005-2736(80)90465-4. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Rilfors L., Lindblom G. Metabolic changes of membrane lipid composition in Acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: arguments for effects on lipid packing. Biochemistry. 1986 Nov 18;25(23):7511–7517. doi: 10.1021/bi00371a038. [DOI] [PubMed] [Google Scholar]