Abstract
Intercellular adhesion molecule 1 (ICAM-1) is an endothelial cell adhesion molecule implicated in cerebral malaria. We investigated whether fibrinogen affects Plasmodium falciparum binding to ICAM-1, as the ICAM-1 binding sites of P. falciparum and fibrinogen overlap. We show that fibrinogen dramatically reduces P. falciparum adhesion to ICAM-1 under flow conditions.
Plasmodium falciparum infection can result in severe anemia, organ failure, cerebral malaria, and coma and can eventually cause death. The unique ability of P. falciparum-infected red blood cells (IRBC) to sequester in the small blood vessels of major organs in humans is thought to be a major contributory factor leading to these extreme complications and is supported by evidence obtained at autopsy (23). Several P. falciparum-derived molecules have been implicated in mediating IRBC adhesion, although not all have been shown to do so conclusively (for a review, see reference 19). Of these molecules, P. falciparum erythrocyte membrane protein 1 (PfEMP1) (3, 18) has attracted the most attention, as cleavage of PfEMP1 from the red blood cell (RBC) surface by trypsin inhibits adhesion (18) and a number of receptor binding sites have been mapped to this molecule (2). Numerous endothelial cell surface molecules bind IRBC (reviewed by Ho and White [14]). However, binding to intercellular adhesion molecule 1 (ICAM-1), one of the few molecules whose expression is up regulated in malaria (5, 25), has been found to be greatest in parasite isolates obtained from patients with cerebral malaria (20). As the ICAM-1 binding sites of PfEMP1 and fibrinogen (Fg) overlap (11), Fg may affect IRBC binding to ICAM-1 and thereby affect malaria pathogenesis.
We used two lines of P. falciparum, A4 (24) and ItG (21), to investigate whether Fg (at levels comparable to those observed in patients with malaria [15]) alters IRBC binding to ICAM-1 under flow conditions. Parasites were cultured in RPMI 1640 medium (supplemented with 37.5 mM HEPES, 7 mM d-glucose, 6 mM NaOH, 25 μg of gentamicin sulfate/ml, 2 mM l-glutamine, and 10% human serum) at a pH of 7.2 in a gas mixture of 96% nitrogen, 3% carbon dioxide, and 1% oxygen. On day 4, parasites were synchronized with sorbitol, and prior to use on day 5 or 7, parasites were washed twice in binding buffer (RPMI 1640 medium supplemented with 6 mM glucose, pH 7.2). Cultures were resuspended to 1% hematocrit (Coulter Counter) and 3% parasitemia (Giemsa staining). For the flow assay, microslides were coated with ICAM-1-Fc (10 μg/ml), blocked overnight at 4°C, and then incubated with varying concentrations of human Fg or fibronectin. For the flow experiments (7), we used a flow rate that yielded a wall shear stress of 0.05 Pa, which has been used widely to mimic wall shear stresses in capillaries. IRBC suspensions were allowed to flow over the microslides for a total of 5 min, with the recording of six separate fields on the microslide (10-s clips) carried out at the fifth minute at ×2,000 magnification. Once recording was finished, binding buffer was used to remove unbound RBC and the number of stationary IRBC was counted in six separate areas on the microslide at ×300 magnification, from which the number of stationary IRBC per square millimeter was calculated. Examination at ×2,000 magnification revealed that almost all adherent cells (>99%) were parasitized. The velocity of all adherent IRBC captured in the recording at 5 min was calculated and analyzed. From the velocity data, the actual number of rolling IRBC could be determined if a velocity of <15 μm/s was deemed to be stationary. Statistical significance was determined by the Tamhane T-2 test, and differences were regarded as significant if P was <0.05.
A4 and ItG binding to ICAM-1.
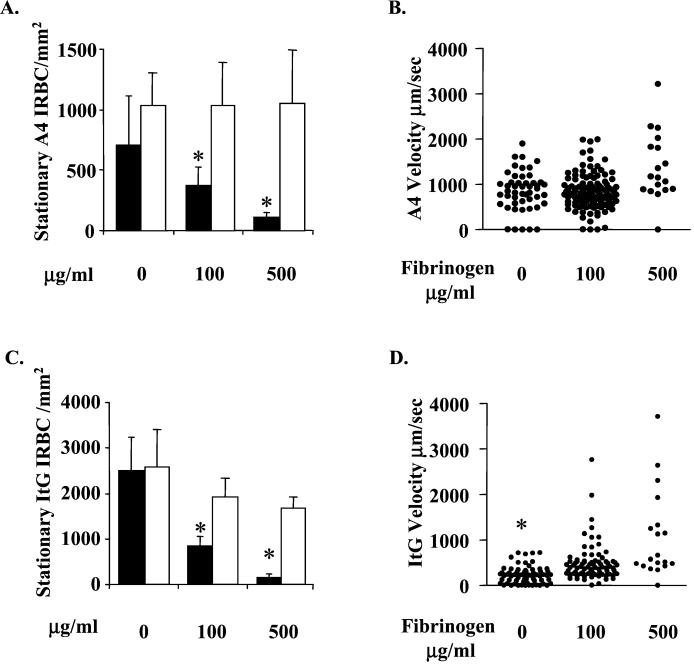
Weak A4 binding to ICAM-1 resulted in little stationary adhesion; however, the majority of A4 adhesive interactions were rolling adhesions (Fig. 1A and B). In contrast, the stronger binding of ItG IRBC to ICAM-1 generated many more stationary ItG and rolling velocities were much lower (Fig. 1C and D). Differences between the adhesion of A4 and ItG to ICAM-1 were highlighted by significantly greater numbers of stationary ItG which bind ICAM-1 (P < 0.003 in Fig. 1C) and the significantly lower rolling velocity of ItG on ICAM-1 (P < 0.001 in Fig. 1D). Our results agree not only with previous research investigating A4 and ItG IRBC binding to ICAM-1 (1) but with the variable binding behavior of field isolates (8). This variation in binding behavior between parasite isolates and laboratory lines may be explained by the differential adhesion properties of the variant surface proteins expressed.
FIG. 1.
Fg inhibits A4 and ItG binding to ICAM-1. Experimental details are as described in the text. Briefly, ICAM-1-coated (10 μg/ml) microslides were incubated with varying concentrations of Fg or fibronectin diluted in binding buffer for 1 h at 37°C. A4 and ItG IRBC (3% parasitemia, 1% hematocrit) were then allowed to flow over the microslides at a wall shear stress of 0.05 Pa. (A and C) The means and standard errors of the means of stationary A4 (A) and ItG (C) IRBC per square millimeter from six different fields in three separate experiments using ICAM-1-coated microslides incubated with either Fg (filled bars) or fibronectin (open bars). (B and D) Rolling velocity (micrometers per second) of individual A4 (B) and ItG (D) IRBC from all three Fg experiments. ∗, statistically significant at P < 0.05.
Fg inhibits A4 and ItG binding to ICAM-1.
Binding of Fg in high concentrations to ICAM-1 blocked A4 and ItG binding, demonstrated by the significantly reduced numbers of stationary IRBC bound to ICAM-1 (P < 0.001 in both Fig. 1A and C), by a general increase in IRBC rolling velocity on ICAM-1 (Fig. 1B; P < 0.008 in Fig. 1D), and by a reduction in the numbers of IRBC which roll on ICAM-1 (determined during rolling velocity analysis). Repeating these experiments with fibronectin had little or no effect upon A4 or ItG IRBC binding to ICAM-1 with regard to stationary adhesion (Fig. 1A and C, respectively), rolling velocity, or the number of rolling IRBC (data not shown). Therefore, as Fg prevents the adhesion of IRBC to ICAM-1, it may reduce IRBC sequestration and thereby reduce malaria pathogenesis.
Little research has directly addressed the relationship between Fg and malaria pathogenesis, although hypofibrinogenemia in P. falciparum malaria has been observed (15). However, this study did not directly compare Fg levels to parasitemia and the clinical severity of acute malaria cases was unclear. Our results suggest that hyperfibrinogenemia may inhibit A4- and ItG-like adhesion to ICAM-1, thereby protecting against severe malaria. Conversely, hypofibrinogenemia may augment susceptibility to severe malaria. It is of interest, therefore, that a single natural polymorphism (K29 M, termed ICAM-1kilifi) in the PfEMP1 binding site on ICAM-1 inhibits Fg binding to ICAM-1 (10). If ICAM-1-bound Fg does prevent IRBC sequestration, then disease severity should be increased in ICAM-1kilifi homozygotes, and indeed a Kenyan study found ICAM-1kilifi-homozygous children significantly more susceptible to cerebral malaria (12). However, in Gabon, the incidence of severe malaria was reduced in ICAM-1kilifi-homozygous children (17), although disease states were not categorized into cerebral malaria and severe malaria, and a Gambian study found no effect (4). The results of these contrasting studies may reflect several differences (9), including P. falciparum line diversity, as coinfection with more than one parasite line has been shown (6, 16) and binding phenotypes vary between lines (for a review, see reference 19). Matters are complicated by the fact that Fg enhances ICAM-1 expression on human endothelial cells (13, 22), and so Fg may exacerbate disease severity in P. falciparum malaria by increasing ICAM-1 expression on endothelium.
While ICAM-1-bound Fg dramatically affects A4 and ItG adhesion on ICAM-1, at present it is unclear whether Fg alters ICAM-1 conformation or blocks the PfEMP1 binding site. Fg does not block the RBC surface, however, as adding Fg to the parasite suspension did not affect IRBC adhesion (data not shown). Conformational change of ICAM-1kilifi, shown by the loss of the BBA4 monoclonal antibody epitope (10), may explain previous research which shows disruption of A4, but not ItG, binding to ICAM-1kilifi (1). However, our research shows that ICAM-1-bound Fg disrupts the binding of both A4 and ItG to ICAM-1 and, as such, binding site blockade would be a more likely explanation. The relevance of this in disease, including the complex role which Fg plays at a cellular level with regard to ICAM-1, will form the basis of further investigations.
Acknowledgments
We thank Ian Hastings (Liverpool School of Tropical Medicine) for advice on statistical analysis. Many thanks to Gareth Turner and Sue Adams (Nuffield Department of Clinical Laboratory Science, Oxford, United Kingdom) for all their help and support, which enabled us to carry out flow adhesion work in Liverpool.
This research was funded by project grants to A. G. Craig from the Wellcome Trust and the European Union (EU grant QLK2/CT/2000/00109).
Editor: B. B. Finlay
REFERENCES
- 1.Adams, S., G. D. Turner, G. B. Nash, K. Micklem, C. I. Newbold, and A. G. Craig. 2000. Differential binding of clonal variants of Plasmodium falciparum to allelic forms of intracellular adhesion molecule 1 determined by flow adhesion assay. Infect. Immun. 68:264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruch, D. I. 1999. Adhesive receptors on malaria-parasitized red cells. Bailliere's Best Pract. Res. Clin. Haematol. 12:747-761. [DOI] [PubMed] [Google Scholar]
- 3.Baruch, D. I., J. A. Gormely, C. Ma, R. J. Howard, and B. L. Pasloske. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 93:3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy, R., D. Kwiatkowski, and A. V. Hill. 1998. Absence of an association between intercellular adhesion molecule 1, complement receptor 1 and interleukin 1 receptor antagonist gene polymorphisms and severe malaria in a West African population. Trans. R. Soc. Trop. Med. Hyg. 92:312-316. [DOI] [PubMed] [Google Scholar]
- 5.Berendt, A. R., D. L. Simmons, J. Tansey, C. I. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. C., M. R. Galinski, J. W. Barnwell, C. A. Donnelly, M. Walmsley, M. P. Alpers, D. Walliker, and K. P. Day. 2000. Genetic diversity and dynamics of Plasmodium falciparum and P. vivax populations in multiply infected children with asymptomatic malaria infections in Papua New Guinea. Parasitology 121:257-272. [DOI] [PubMed] [Google Scholar]
- 7.Cooke, B. M., A. R. Berendt, A. G. Craig, J. MacGregor, C. I. Newbold, and G. B. Nash. 1994. Rolling and stationary cytoadhesion of red blood cells parasitised by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br. J. Haematol. 87:162-170. [DOI] [PubMed] [Google Scholar]
- 8.Cooke, B. M., S. Morris-Jones, B. M. Greenwood, and G. B. Nash. 1995. Mechanisms of cytoadhesion of flowing, parasitized red blood cells from Gambian children with falciparum malaria. Am. J. Trop. Med. Hyg. 53:29-35. [PubMed] [Google Scholar]
- 9.Craig, A., I. Hastings, A. Pain, and D. J. Roberts. 2001. Genetics and malaria—more questions than answers. Trends Parasitol. 17:55-56. [DOI] [PubMed] [Google Scholar]
- 10.Craig, A. G., D. Fernandez-Reyes, M. Mesri, A. McDowall, L. H. Miller, D. C. Altieri, N. Hogg, and C. I. Newbold. 2000. A functional analysis of a natural variant of intercellular adhesion molecule-1 (ICAM-1kilifi). Hum. Mol. Genet. 9:525-530. [DOI] [PubMed] [Google Scholar]
- 11.Duperray, A., L. R. Languino, J. Plescia, A. McDowall, N. Hogg, A. G. Craig, A. R. Berendt, and D. C. Altieri. 1997. Molecular identification of a novel fibrinogen binding site on the first domain of ICAM-1 regulating leukocyte-endothelium bridging. J. Biol. Chem. 272:435-441. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Reyes, D., A. G. Craig, S. A. Kyes, N. Peshu, R. W. Snow, A. R. Berendt, K. Marsh, and C. I. Newbold. 1997. A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum. Mol. Genet. 6:1357-1360. [DOI] [PubMed] [Google Scholar]
- 13.Harley, S. L., J. Sturge, and J. T. Powell. 2000. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20:652-658. [DOI] [PubMed] [Google Scholar]
- 14.Ho, M., and N. J. White. 1999. Molecular mechanisms of cytoadherence in malaria. Am. J. Physiol. 276:C1231-C1242. [DOI] [PubMed] [Google Scholar]
- 15.Jimmy, E. O., I. Saliu, I. Okpala, O. Walker, A. Sowumni, O. G. Ademowo, and E. M. Essien. 1995. Effect of Plasmodium falciparum malaria on plasma fibrinopeptide-A (FpA) concentration. Cent. Afr. J. Med. 41:124-127. [PubMed] [Google Scholar]
- 16.Khattab, A., J. Kun, P. Deloron, P. G. Kremsner, and M. Q. Klinkert. 2001. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J. Infect. Dis. 183:1165-1169. [DOI] [PubMed] [Google Scholar]
- 17.Kun, J. F., J. Klabunde, B. Lell, D. Luckner, M. Alpers, J. May, C. Meyer, and P. G. Kremsner. 1999. Association of the ICAM-1Kilifi mutation with protection against severe malaria in Lambarene, Gabon. Am. J. Trop. Med. Hyg. 61:776-779. [DOI] [PubMed] [Google Scholar]
- 18.Leech, J. H., J. W. Barnwell, L. H. Miller, and R. J. Howard. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 159:1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newbold, C., A. Craig, S. Kyes, A. Rowe, D. Fernandez-Reyes, and T. Fagan. 1999. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int. J. Parasitol. 29:927-937. [DOI] [PubMed] [Google Scholar]
- 20.Newbold, C., P. Warn, G. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 57:389-398. [DOI] [PubMed] [Google Scholar]
- 21.Ockenhouse, C. F., M. Ho, N. N. Tandon, G. A. Van Seventer, S. Shaw, N. J. White, G. A. Jamieson, J. D. Chulay, and H. K. Webster. 1991. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J. Infect. Dis. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 22.Qi, J., D. L. Kreutzer, and T. H. Piela-Smith. 1997. Fibrin induction of ICAM-1 expression in human vascular endothelial cells. J. Immunol. 158:1880-1886. [PubMed] [Google Scholar]
- 23.Riganti, M., E. Pongponratn, T. Tegoshi, S. Looareesuwan, B. Punpoowong, and M. Aikawa. 1990. Human cerebral malaria in Thailand: a clinico-pathological correlation. Immunol. Lett. 25:199-205. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner, G. D., H. Morrison, M. Jones, T. M. Davis, S. Looareesuwan, I. D. Buley, K. C. Gatter, C. I. Newbold, S. Pukritayakamee, B. Nagachinta, et al. 1994. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145:1057-1069. [PMC free article] [PubMed] [Google Scholar]