Abstract
Pseudomonas aeruginosa clinical cystic fibrosis isolate CHA was mutagenized with Tn5Tc to identify new genes involved in type III secretion system (TTSS)-dependent cytotoxicity toward human polymorphonuclear neutrophils. Among 25 mutants affected in TTSS function, 14 contained the insertion at different positions in the aceAB operon encoding the PDH-E1 and -E2 subunits of pyruvate dehydrogenase. In PDH mutants, no transcriptional activation of TTSS genes in response to calcium depletion occurred. Expression in trans of ExsA restored TTSS function and cytotoxicity.
Pseudomonas aeruginosa is a major gram-negative opportunistic pathogen responsible for both acute and chronic infections. Chronic P. aeruginosa colonization of the airways of cystic fibrosis (CF) patients and subsequent acute infections are the leading cause of morbidity in CF. The adaptation of P. aeruginosa to the environment of CF lungs is accompanied by the synthesis of diverse virulence factors comprising various exoproteins and mucoid exopolysaccharides (11). One of the reasons for the secretion of virulence factors is to allow the bacteria to avoid the host defense mechanism, the main line of which comprises the bactericidal activity of polymorphonuclear neutrophils (PMNs). The type III secretion system (TTSS) is a recently identified virulence determinant of P. aeruginosa (19). It encodes on the order of 20 proteins, including (i) components of a secretory apparatus, (ii) components of machinery devoted to the direct translocation of effectors into the host cell cytoplasm, and (iii) four effectors, ExoS, -T, -U, and -Y, thought to alter normal host cell processes (10). It has recently been shown that some CF isolates of P. aeruginosa are able to resist the bactericidal activity of PMNs and to induce rapid TTSS-dependent oncotic cell death of both PMNs and macrophages (1-4, 15). The first step of the activation of the expression of TTSS genes in P. aeruginosa is the upregulation of the transcription of the exsA gene (part of the exsCBA operon) in response to different stimuli such as calcium depletion in vitro or target cell contact in vivo (9). In a previous study we showed that expression of ExsA in trans was sufficient to activate in vitro secretion and ex vivo cytotoxicity toward phagocytes in noncytotoxic CF isolates (2). Here we used large-scale genetic screening to identify new genes required for cytotoxicity.
The bacterial strains and plasmids used in this study are listed in Table 1. The parental CHA strain has previously been characterized as cytotoxic and able to induce rapid TTSS-dependent oncosis of PMNs and J774 macrophages (1-4). This strain produces the type III effectors ExoS and ExoT but not ExoU because of the absence of the exoU gene in the CHA genome (1). Transposon mutants of the cytotoxic P. aeruginosa CHA strain were generated by bacterial conjugation. A conjugation-proficient suicide plasmid, pUTTn5-Tetr was introduced into the P. aeruginosa strain CHA by triparental mating (8 h at 37°C) using the helper plasmid pRK2013. Mating mixtures were recovered and resuspended in 10 ml of sterile 10 mM MgSO4 before plating on Vogel-Bonner minimal medium (17) agar plates containing tetracycline (100 μg ml−1) to counterselect against Escherichia coli donor strains. Growth on Vogel-Bonner minimal medium allowed us to eliminate auxotrophic mutants, which may appear noncytotoxic. Southern blot analysis of chromosomal DNA isolated from randomly picked mutants was performed to verify that most of the obtained mutants resulted from independent transposition events rather than from replication of siblings. Only a single and different PstI restriction fragment from each random mutant hybridized to a probe derived from PCR labeling of the tetracycline gene, amplified using primers 5′TAATGCGGTAGTTTATCACAG and 5′ACTGGCGATGCTGTCGGAATG (GenBank accession number X67018), indicating that only a single transposon insertion event occurred in each mutant. For the cytotoxicity assay, the human PMNs were obtained as described previously (1). The assay conditions for P. aeruginosa cytotoxicity on PMNs were adapted from previously reported conditions (1) for 96-well microplates. In each well, 106 PMNs were infected with 107 bacteria. For each experiment a positive control corresponding to the parental cytotoxic CHA strain and a negative control using its ExsA isogenic, noncytotoxic CHA-D1 mutant were added (1). Cytotoxicity was quantified spectrophotometrically by measuring the release of lactate dehydrogenase (LDH) (1, 4) in the infection medium 3 h after addition of bacteria. The PMNs released less than 10% LDH in uninfected conditions whereas infections with the CHA strain resulted in an eightfold increase in LDH release. The screening of 5,070 mutants on PMNs allowed us to select 53 mutants yielding less than 30% of the cytotoxicity of CHA. These experiments were performed in triplicate at least three times with several different PMN preparations.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties or genotypea | Source or reference |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| CHA | Mucoid CF isolate, cytotoxic | 5 |
| CHA-D1 | exsA::Gm mutant of CHA, noncytotoxic | 1 |
| E. coli DH5α | Φ80dlacZΔM15 F−endA1 hsdR17 (rk− mk+) supE44 thi-1 λ−recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 | Eurogentec |
| Plasmids | ||
| pUC18 | Apr, cloning vector | New England Biolabs |
| PRK2013 | Kmr, mobilizing plasmid helper | 8 |
| pUTTn5-Tetr | Tetr, containing Tn5 transposon | 6 |
| pIApC | Cbr Apr, containing gfpmut3 gene under pexsC promoter | 3 |
| pAG710 | Cbr Apr, containing aceB gene under constitutive promoter | This work; reference 1 |
| pDD2 | pUCP20-derived plasmid containing exsA gene under a constitutive promoter | 3 |
Apr, ampicillin resistance; Tetr, tetracycline resistance; Kmr, kanamycin resistance; Cbr, carbenicillin resistance.
Each noncytotoxic mutant was checked for the functionality of TTSS by examining the protein secretion profile of the culture supernatant with or without induction of the secretion system by calcium depletion (19). Among the several proteins secreted when the CHA strain is grown under calcium-depleted conditions, four major proteins have been shown to be characteristic of TTSS: ExoS, ExoT, PopB, and PopD (1, 4) (Fig. 1A). Twenty-five of the mutants were found to be unable to give a secreted protein profile similar to that of CHA (Fig. 1 and Table 2). The mutants affected in TTSS function were genetically characterized by sequencing the Tn5 transposon insertion site. Briefly, P. aeruginosa chromosomal DNA was isolated, digested with the restriction enzyme PstI, and then cloned in pUC18. Plasmids from the selected colonies that grew on 10-μg ml−1 tetracycline Luria-Bertani (LB) medium were sequenced (Genome Express, Grenoble, France) with a primer hybridizing the 3′ end of the transposon (5′GCCGGATCCGCCGGTAGAC) (6). Comparison of the nucleotide sequences with the data bank using the Blast 2.0 program (www.ncbi.nlm.nih.gov/blast) and the finished genomic sequence of P. aeruginosa (16) revealed possible functions for all the genes harboring transposon insertions (Table 2). The number of occurrences for each of the inactivated genes is indicated. For mutants 12, 21, 22, 23, 26, 43, and 46, ExoS and ExoT bands were completely absent from the protein profile (Fig. 1A). However, among these mutants, only 12 and 22 had an insertion in a known TTSS gene, exsD and pscL, respectively. We cannot exclude that Tn5 insertions have a polar effect on downstream genes, but the results presented here suggest the involvement of new genes in TTSS functioning. We will describe only two of them (aceA and aceB) further in this work and will perform complementation with them. Mutant 37, inactivated in pcrV, secreted ExoS and ExoT but failed to secrete PopB and PopD. Mutant 43 had transposon insertion in the ppX gene encoding an exopolyphosphatase. In P. aeruginosa, the genes encoding polyphosphate kinase and exopolyphosphate phosphatase, which are involved in polyphosphate synthesis and degradation, respectively, are contiguous and convergently transcribed (20). Mutant 43 (ppX) presents a clear defect in the secretion of type III effectors (Fig. 1). The intracellular level of inorganic polyphosphate is regulated through the action of the exopolyphosphate kinase and phosphatase. Many studies have been performed to characterize the effects of inactivation of polyphosphate kinase, which is involved not only in the twitching motility phenotype, but also in biofilm development, quorum sensing, and the virulence of P. aeruginosa (13). The work described above suggests that polyphosphate phosphatase could also be involved in TTSS functioning. Mutant 46 is inactivated in the dsbA gene encoding a periplasmic thiol/disulfide oxidoreductase known to have pleiotropic effects in E. coli and to affect the periplasmic maturation of TTSS proteins in Shigella flexneri (7). It has to be noticed that the main protein band visible on the profile of mutant 46 is not PopD: the band actually migrates below the level of PopD and was not seen in any of the experiments done with the different dsbA mutants. The mutants in aceB (pyruvate dehydrogenase [PDH]-E2 subunit, dihydrolipoamide acetyltransferase) and aceA (PDH-E1 subunit, PDH) have a secretion profile containing the four characteristic proteins but in much lower amounts (Fig. 1). These PDH mutants correspond to the majority (14 of 25) of the isolated TTSS-deficient mutants. The transposon insertion sites of the aceA or aceB mutants determined by sequencing were located at different positions throughout the aceAB operon, indicating the absence of a true hot spot of transposition. We focused on two representatives of these mutants (9 and 44) in order to analyze why an insertion in the aceAB operon results in a defect in TTSS-dependent cytotoxicity.
FIG. 1.
Analysis of TTSS function by electrophoretic analysis of P. aeruginosa proteins secreted into extracellular media under TTSS-inducing calcium depletion conditions (5 mM EGTA, 20 mM MgCl2). Secreted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by silver staining. In each lane, the same amount of supernatants collected from culture grown to the same optical density at 600 nm was used. (A) Profile obtained with CHA, CHA-D1, and noncytotoxic mutants cultured in inducing conditions. (B) Profile obtained either in noninducing (−) or inducing (+) conditions with mutant 44 (aceA) and mutant 9 (aceB). (ExsA), the strain contained the pDD2 plasmid expressing ExsA. The CHA wild type was used as a control for both panels.
TABLE 2.
TTSS-deficient noncytotoxic mutants
| Mutant | Gene(s) | GenBank accession no. | Cytotoxicity (% of CHA)a | No. of gene occurence | TTSS functionb |
|---|---|---|---|---|---|
| 12 | exsD | PA1714 | 3 | 1 | − |
| 21 | phoA | PA3296 | 3 | 1 | − |
| 22 | pscL | PA1725 | 3 | 1 | − |
| 23 | rpoN | PA4462 | 2 | 1 | − |
| 26 | mreC | PA4480 | 8 | 1 | − |
| 43 | ppx | PA5241 | 10 | 1 | − |
| 36, 37 | pcrV | PA1706 | 8 | 2 | −(PopB/D) |
| 27, 32, 46 | dsbA | PA5489 | 2 | 3 | − |
| 24, 25, 44, 52 | aceA | PA5015 | 1 | 4 | Small amount |
| 9, 11, 13, 39, 42, 45, 47, 50, 53, 54 | aceB | PA5016 | 3 | 10 | Small amount |
| 44 (pDD2) | aceA, with constitutive exsA | 80 | + | ||
| 9 (pDD2) | aceB, with constitutive exsA | 80 | + | ||
| 9 (pAG710) | aceB, complemented aceB | 70 | + |
For further details, see the text. Values are means of three experiments.
+, TTSS function is present; −, no TTSS function.
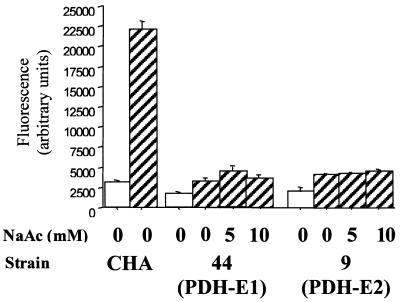
We previously showed that expression of ExsA in trans confers TTSS-dependent cytotoxicity on noncytotoxic P. aeruginosa CF isolates (3). We transformed mutant PDH-E1 and -E2 with the plasmid pDD2 carrying the constitutively expressed exsA gene (3). The cytotoxicity level of the PDH mutants containing pDD2 reached 80% (±11%) of the level of the wild-type CHA strain (Table 2). Furthermore, sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of supernatants of PDH mutants and PDH mutants containing pDD2 confirmed a partial restoration of the TTSS function (Fig. 1B). Taken together, these results suggest that in the PDH mutants exsA expression is not induced, which does not allow the TTSS to function efficiently. To verify this hypothesis we performed transcriptional analysis in PDH mutants containing the plasmid pIApC, which harbors a transcriptional fusion of the exsCBA promoter (pC) with the gfp gene (3). Strains were cultured under noninduced and TTSS-inducing conditions (5 mM EGTA, 20 mM MgCl2) and fluorescence was measured as described previously (3). Both the aceA and aceB mutants were unable to activate transcription from the exsCBA promoter in response to calcium depletion (Fig. 2). Previous studies concerning PDH mutants of P. aeruginosa have shown that they require 5 mM acetate for normal aerobic growth (12). We checked the effect of supplementation with 5 and 10 mM sodium acetate, pH 7.2, in LB culture on the level of expression of the exsCBA operon and cytotoxicity. Although able to grow as fast as the CHA strain when supplemented with 5 mM acetate (data not shown), PDH mutants were still unable to activate transcription of the exsCBA operon upon calcium limitation (Fig. 2). Furthermore, cytoxicity was not improved by acetate supplementation (data not shown). Although we cannot exclude that the mutation in the aceAB operon could lead to a more global effect on cellular physiology, the results obtained with growth on acetate suggest that the metabolic effect of PDH inactivation is not responsible for the observed phenotype. In order to eliminate polar effects of the transposon mutation we performed complementation of the PDH-E2 mutants with the aceB gene isolated from CHA genomic DNA. Plasmid pAG710 was obtained by amplifying aceB using oligos ABX1 (5′-TATATCTAGAGCGGTGGTGGCACGTAA) and ABX2 (GAGATCTAGATTCGCCGAGCCGTCAC) (XbaI sites are shown in bold). The PCR contained genomic DNA from CHA as template and we used Pfu polymerase. The obtained 1.8-kb fragment was digested with XbaI and cloned in the proper orientation behind the X12 promoter in pIAX12 (1). Plasmid pAG710 was introduced in PDH-E2 mutants by electroporation. The restoration of both cytotoxicity toward PMNs to 70% (±9%) of the level of the wild-type CHA strain (Table 2) and the in vitro TTSS secretion was noticed, allowing us to eliminate a possible polar effect of the Tn5Tc insertion on downstream genes. No complementation of the aceA mutant was noticed with the aceB genes (data not shown).
FIG. 2.
GFP fluorescence in CHA and PDH mutants transformed with plasmid pIApC grown in LB medium (white bars) or in LB medium supplemented with 5 mM EGTA and 20 mM MgCl2 (inducing conditions) (hatched bars). The final concentration of sodium acetate in the culture medium is indicated. Error bars represent the standard deviations. Data are from at least three independent experiments.
In view of our results, we investigated the virulence of the PDH mutants 9 and 44 in a rat model of pneumonia. Ten Sprague-Dawley rats (280 to 300 g) were infected with either the wild-type CHA strain, a CHA-D1 ExsA-deficient mutant (1), PDH mutant 44 (aceA), or PDH mutant 9 (aceB). Rats were briefly anesthetized with ethanol ether. The neck was washed extensively with 70°C ethanol, and an anterior median incision was performed to locate the trachea with forceps. A bacterial inoculum of 2 × 109 bacteria/kg of body weight reconstituted in 0.5 ml/kg in isotonic saline was injected through a needle inserted in the trachea. The skin was sutured up and the rats were returned to their cages. Surviving rats were counted for each mutant at 72 h postinfection. Whereas infection with CHA led to 100% lethality in 72 h, no rat death occurred when infection was performed with the ExsA or PDH mutants.
We showed here that screening of CHA transposon mutants for the loss of cytotoxicity towards PMNs allowed us to identify new genes involved in TTSS function. Most of the transposon insertions were found in the aceAB operon encoding the PDH-E1 and -E2 subunits. In these mutants there is no activation of the transcription of the TTSS regulatory operon exsCBA. The cytotoxicity and the TTSS function are restored by complementation with exsA or aceB. Furthermore, PDH mutants are totally avirulent in a rat model of acute pneumonia. Previous work showed that the E1 or E2 subunits of PDH could play a role in the regulation of gene expression. In Azotobacter vinelandii, the E1 subunit of the PDH is a transcription activator of the NADPH:ferredoxin reductase in response to an oxidative stress provoked by superoxide anions (14). The authors of that report also stated that the PDH-E1 subunits of P. aeruginosa and A. vinelandii share 77% amino acid identity and that the A. vinelandii E1 sequence contains a putative helix-turn-helix motif also present in the P. aeruginosa E1 sequence. P. aeruginosa is likely to be submitted to oxidative stress during the interaction with the PMN; therefore, a transcriptional activation by the PDH-E1 subunit could take place to determine an aggressive reaction towards the source of the oxidative species. In Bacillus thuringiensis, the PDH-E2 acts as a transcription activator for coupling postexponential catabolism changes to the synthesis of the protoxin virulence factors (18). Work is under investigation to characterize the P. aeruginosa signaling pathway that uses the PDH-E1 and/or -E2 subunit to activate TTSS gene expression.
Acknowledgments
This work was supported by grant 98033 from the Association Française de Lutte contre la Mucoviscidose (AFLM) and by grants from DGA (DSP/STTC).
We thank F. Morel (Laboratoire d'Enzymologie, CHU-Grenoble) for whole blood facility and student exchange, A. Colbeau for helpful discussions, and J. Chabert for technical assistance.
Editor: E. I. Tuomanen
REFERENCES
- 1.Dacheux, D., I. Attree, C. Schneider, and B. Toussaint. 1999. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect. Immun. 67:6164-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dacheux, D., J. Goure, J. Chabert, Y. Usson, and I. Attree. 2001. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40:76-85. [DOI] [PubMed] [Google Scholar]
- 4.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delic-Attree, I., B. Toussaint, A. Froger, J. C. Willison, and P. M. Vignais. 1996. Isolation of an IHF-deficient mutant of a Pseudomonas aeruginosa mucoid isolate and evaluation of the role of IHF in algD gene expression. Microbiology 142:2785-2793. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabianek, R. A., H. Hennecke, and L. Thony-Meyer. 2000. Periplasmic protein thiol:disulfide oxidoreductases of Escherichia coli. FEMS Microbiol. Rev. 24:303-316. [DOI] [PubMed] [Google Scholar]
- 8.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 10.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchison, M. L., and J. R. Govan. 1999. Pathogenicity of microbes associated with cystic fibrosis. Microbes Infect. 1:1005-1014. [DOI] [PubMed] [Google Scholar]
- 12.Jeyaseelan, K., and J. R. Guest. 1980. Isolation and properties of pyruvate dehydrogenase complex mutants of Pseudomonas aeruginosa PAO. J. Gen. Microbiol. 120:385-392. [DOI] [PubMed] [Google Scholar]
- 13.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:4885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regnstrom, K., S. Sauge-Merle, K. Chen, and B. K. Burgess. 1999. In Azotobacter vinelandii, the E1 subunit of the pyruvate dehydrogenase complex binds fpr promoter region DNA and ferredoxin I. Proc. Natl. Acad. Sci. USA 96:12389-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 16.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 17.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase in Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 18.Walter, T., and A. Aronson. 1999. Specific binding of the E2 subunit of pyruvate dehydrogenase to the upstream region of Bacillus thuringiensis protoxin genes. J. Biol. Chem. 274:7901-7906. [DOI] [PubMed] [Google Scholar]
- 19.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]
- 20.Zago, A., S. Chugani, and A. M. Chakrabarty. 1999. Cloning and characterization of polyphosphate kinase and exopolyphosphatase genes from Pseudomonas aeruginosa 8830. Appl. Environ. Microbiol. 65:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]