Abstract
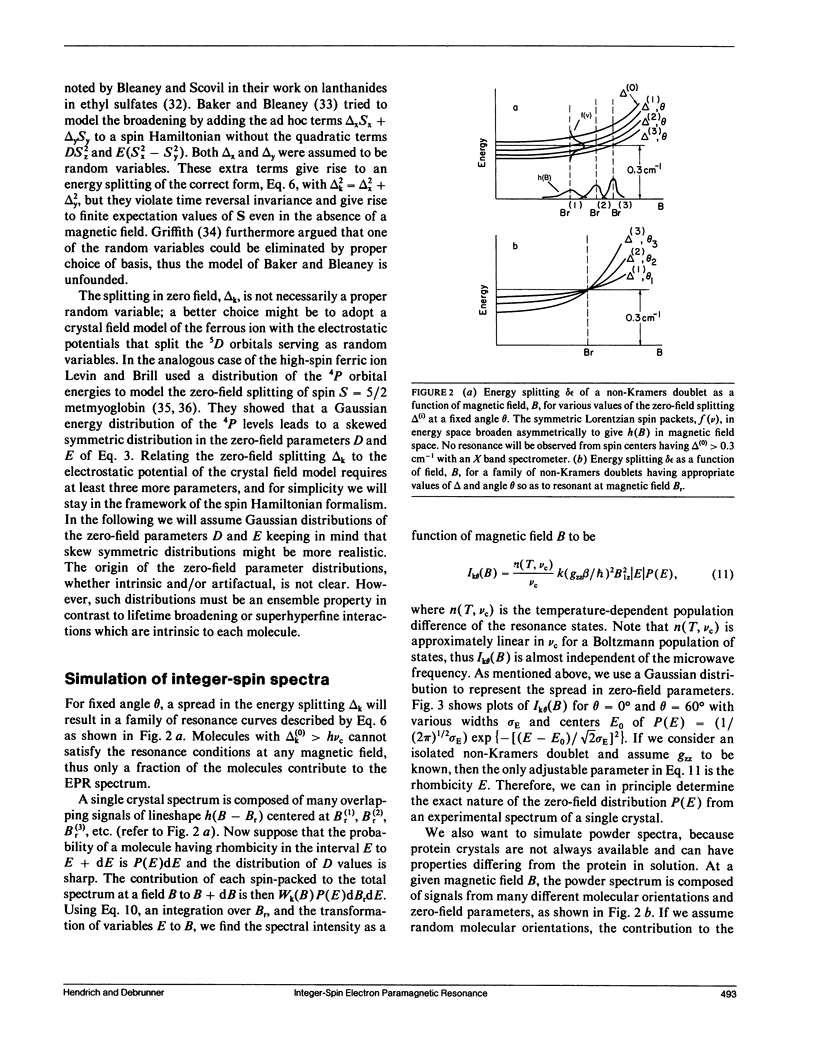
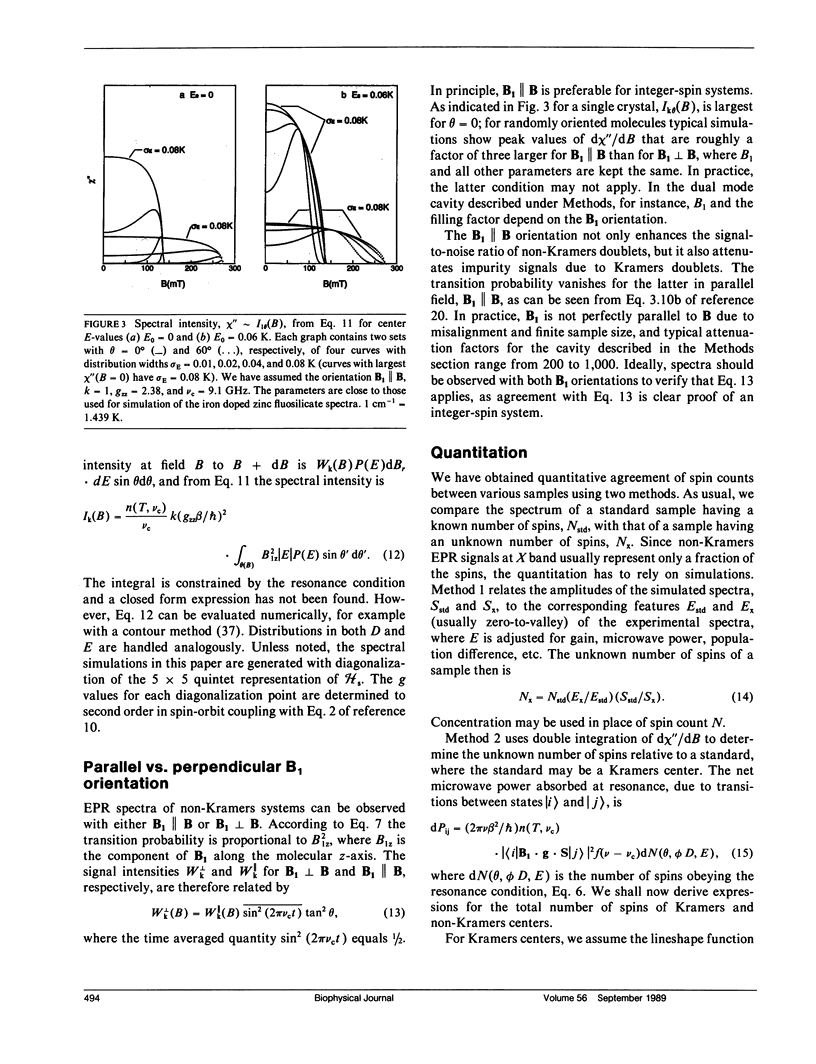
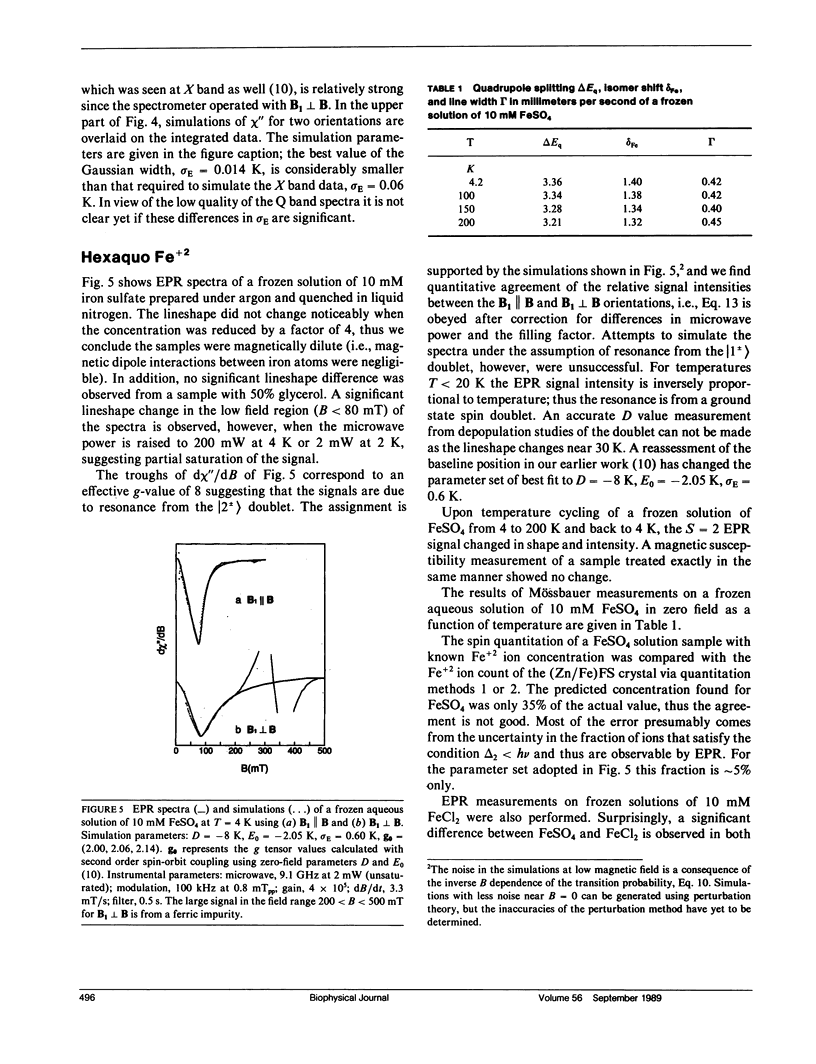
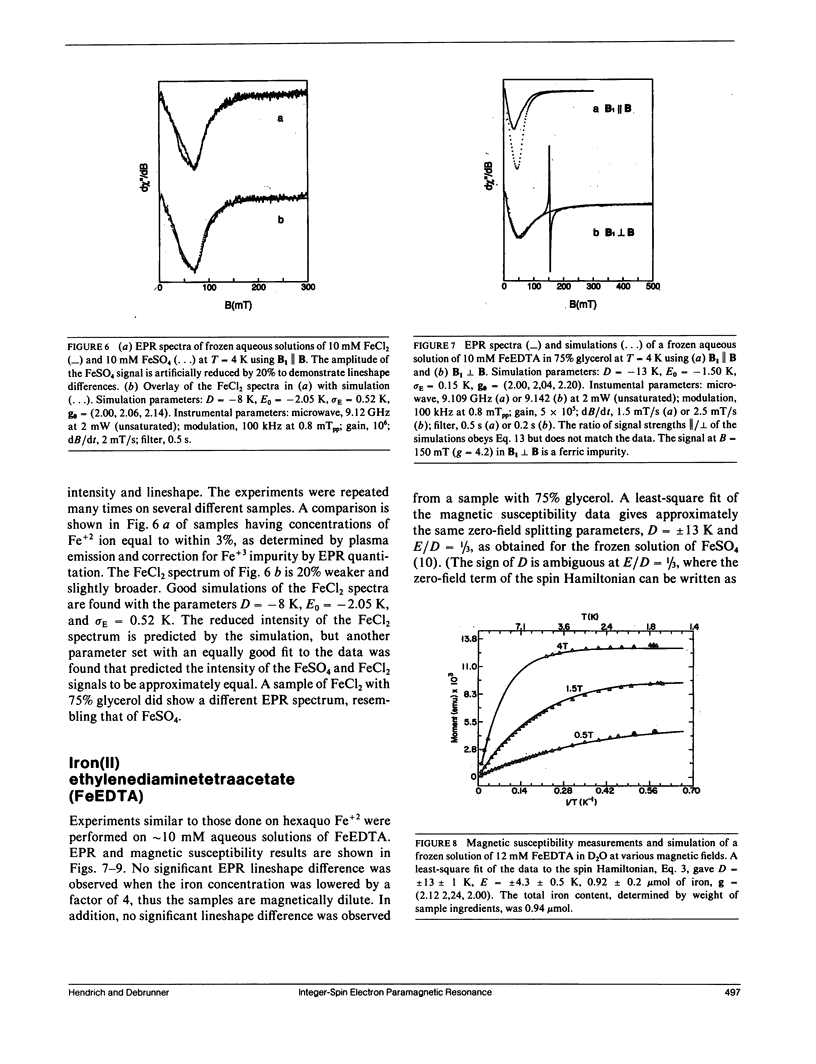
A quantitative interpretation is presented for EPR spectra from integer-spin metal centers having large zero-field splittings. Integer-spin, or non-Kramers, centers are common in metalloproteins and many give EPR signals, but a quantitative understanding has been lacking until now. Heterogeneity of the metal's local environment will result in a significant spread in zero-field splittings and in broadened EPR signals. Using the spin Hamiltonian Hs = S.D.S + beta S.g.B and some simple assumptions about the nature of the zero-field parameter distributions, a lineshape model was devised which allows accurate simulation of single crystal and frozen solution spectra. The model was tested on single crystals of magnetically dilute ferrous fluosilicate. Data and analyses from proteins and active-site models are presented with the microwave field B1 either parallel or perpendicular to B. Quantitative agreement of observed and predicted signal intensities is found for the two B1 orientations. Methods of spin quantitation are given and are shown to predict an unknown concentration relative to a standard with known concentration. The fact that the standard may be either a non-Kramers or a Kramers center is further proof of the model's validity. The magnitude of the splitting in zero magnetic field is of critical importance; it affects not only the chance of signal observation, but also the quantitation accuracy. Experiments taken at microwave frequencies of 9 and 35 GHz demonstrate the need for high-frequency data as only a fraction of the molecules give signals at 9 GHz.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert Y., Banerjee R. Magnetic susceptibility measurements of deoxygenated hemoglobins and isolated chains. Biochim Biophys Acta. 1975 Sep 9;405(1):144–154. doi: 10.1016/0005-2795(75)90324-4. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Baker G. M., Noguchi M., Palmer G. The reaction of cytochrome oxidase with cyanide. Preparation of the rapidly reacting form and its conversion to the slowly reacting form. J Biol Chem. 1987 Jan 15;262(2):595–604. [PubMed] [Google Scholar]
- Brudvig G. W., Stevens T. H., Morse R. H., Chan S. I. Conformations of oxidized cytochrome c oxidase. Biochemistry. 1981 Jun 23;20(13):3912–3921. doi: 10.1021/bi00516a039. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Reed C. A. Syntheses of ferrous-porphyrin complexes. A hypothetical model for deoxymyoglobin. J Am Chem Soc. 1973 Mar 21;95(6):2048–2049. doi: 10.1021/ja00787a075. [DOI] [PubMed] [Google Scholar]
- Dunham W. R., Sands R. H., Shaw R. W., Beinert H. Multiple frequency EPR studies on three forms of oxidized cytochrome c oxidase. Biochim Biophys Acta. 1983 Oct 17;748(1):73–85. doi: 10.1016/0167-4838(83)90029-8. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Surerus K. K., Münck E., Lipscomb J. D. Evidence for a mu-oxo-bridged binuclear iron cluster in the hydroxylase component of methane monooxygenase. Mössbauer and EPR studies. J Biol Chem. 1988 Aug 5;263(22):10553–10556. [PubMed] [Google Scholar]
- Hagen W. R., Dunham W. R., Johnson M. K., Fee J. A. Quarter field resonance and integer-spin/half-spin interaction in the EPR of Thermus thermophilus ferredoxin. Possible new fingerprints for three iron clusters. Biochim Biophys Acta. 1985 Apr 29;828(3):369–374. doi: 10.1016/0167-4838(85)90318-8. [DOI] [PubMed] [Google Scholar]
- Kent T. A., Spartalian K., Lang G., Yonetani T., Reed C. A., Collman J. P. High magnetic field Mössbauer studies of deoxymyoglobin, deoxyhemoglobin, and synthetic analogues. Biochim Biophys Acta. 1979 Oct 24;580(2):245–258. doi: 10.1016/0005-2795(79)90137-5. [DOI] [PubMed] [Google Scholar]
- Kent T., Spartalian K., Lang G., Yonetani T. Mössbauer investigation of deoxymyoglobin in a high magnetic field. Orientation of the electric field gradient and magnetic tensors. Biochim Biophys Acta. 1977 Feb 22;490(2):331–340. doi: 10.1016/0005-2795(77)90008-3. [DOI] [PubMed] [Google Scholar]
- LoBrutto R., Wei Y. H., Yoshida S., Van Camp H. L., Scholes C. P., King T. E. Electron paramagnetic resonance- (EPR-) resolved kinetics of cryogenic nitric oxide recombination to cytochrome c oxidase and myoglobin. Biophys J. 1984 Feb;45(2):473–479. doi: 10.1016/S0006-3495(84)84171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N., Otsuka J., Tasaki A. Fine structure of iron ion in deoxymyoglobin and deoxyhemoglobin. Biochim Biophys Acta. 1971 Apr 27;236(1):222–233. doi: 10.1016/0005-2795(71)90169-3. [DOI] [PubMed] [Google Scholar]
- Nakano N., Otsuka J., Tasaki A. Paramagnetic anisotropy measurements on a single crystal of deoxyhemoglobin. Biochim Biophys Acta. 1972 Sep 29;278(2):355–371. doi: 10.1016/0005-2795(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Roder H., Berendzen J., Bowne S. F., Frauenfelder H., Sauke T. B., Shyamsunder E., Weissman M. B. Comparison of the magnetic properties of deoxy- and photodissociated myoglobin. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2359–2363. doi: 10.1073/pnas.81.8.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Li P. M., Chan S. I. The binuclear site of cytochrome c oxidase. Structural evidence from iron X-ray absorption spectroscopy. Ann N Y Acad Sci. 1988;550:53–58. doi: 10.1111/j.1749-6632.1988.tb35322.x. [DOI] [PubMed] [Google Scholar]
- Steffens G. C., Biewald R., Buse G. Cytochrome c oxidase is a three-copper, two-heme-A protein. Eur J Biochem. 1987 Apr 15;164(2):295–300. doi: 10.1111/j.1432-1033.1987.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Stevens T. H., Martin C. T., Wang H., Brudvig G. W., Scholes C. P., Chan S. I. The nature of CuA in cytochrome c oxidase. J Biol Chem. 1982 Oct 25;257(20):12106–12113. [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- Wagner G. C., Kassner R. J. Spectroscopic properties of ferrous heme complexes of sterically hindered ligands. Biochim Biophys Acta. 1975 Jun 12;392(2):319–327. doi: 10.1016/0304-4165(75)90013-6. [DOI] [PubMed] [Google Scholar]


