Abstract
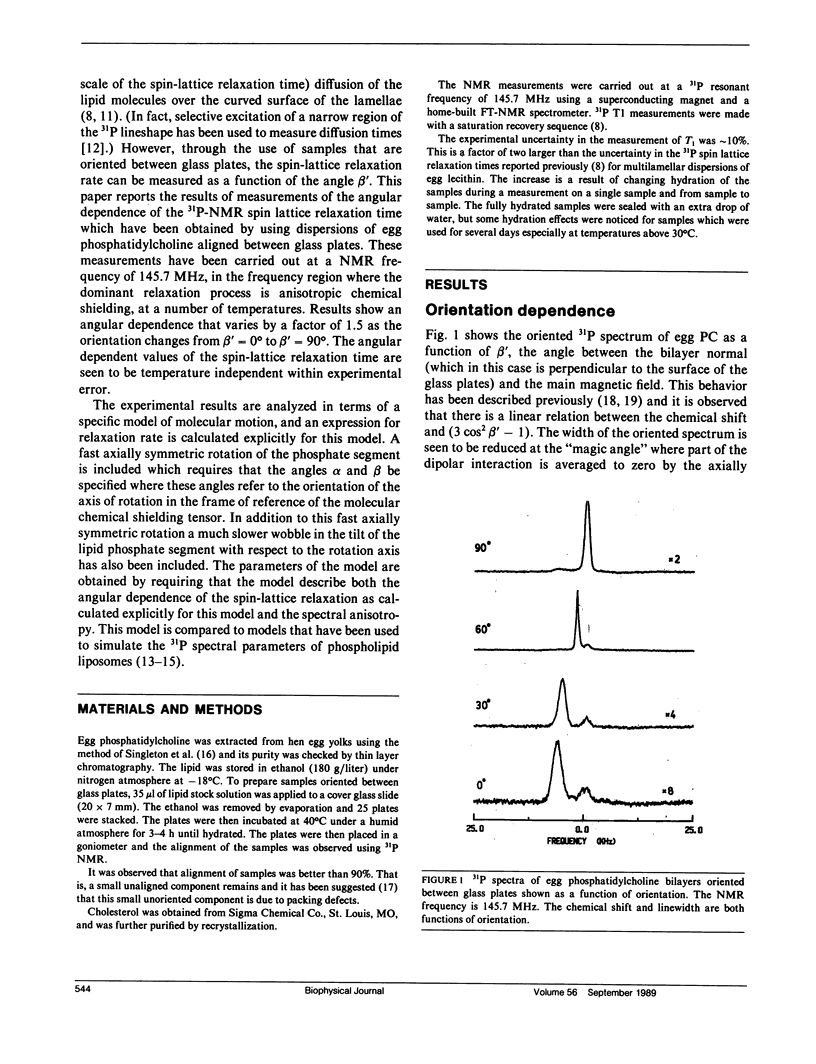
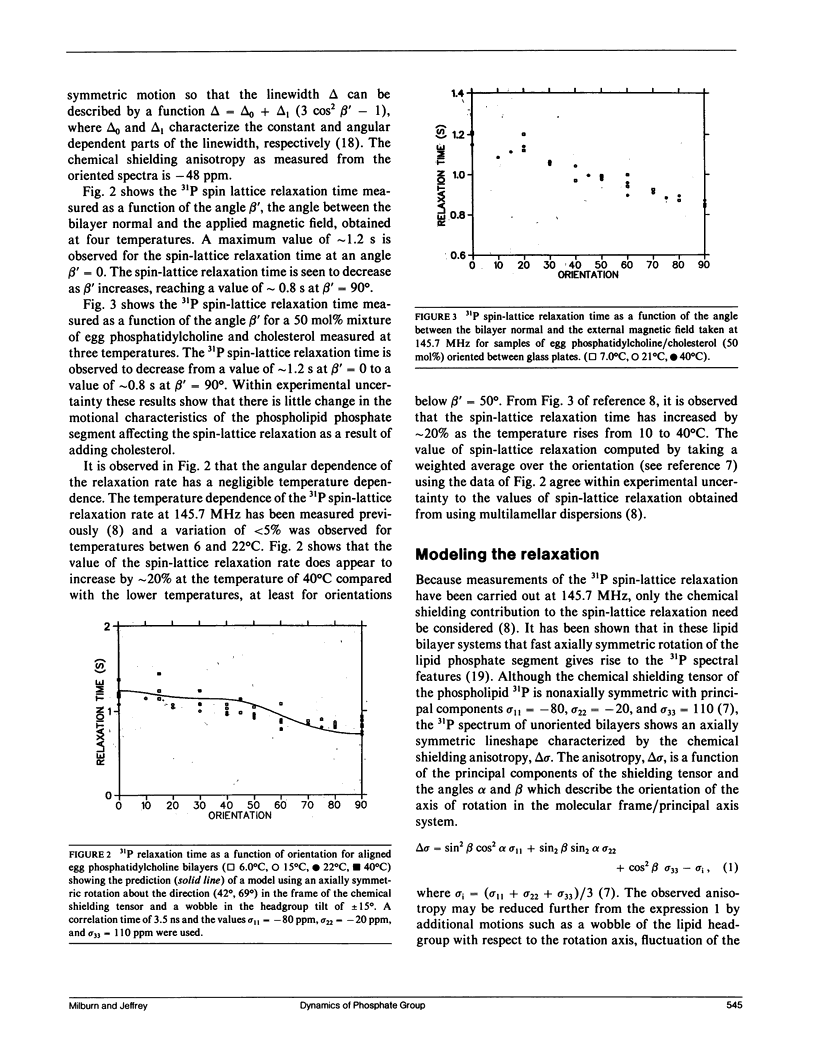
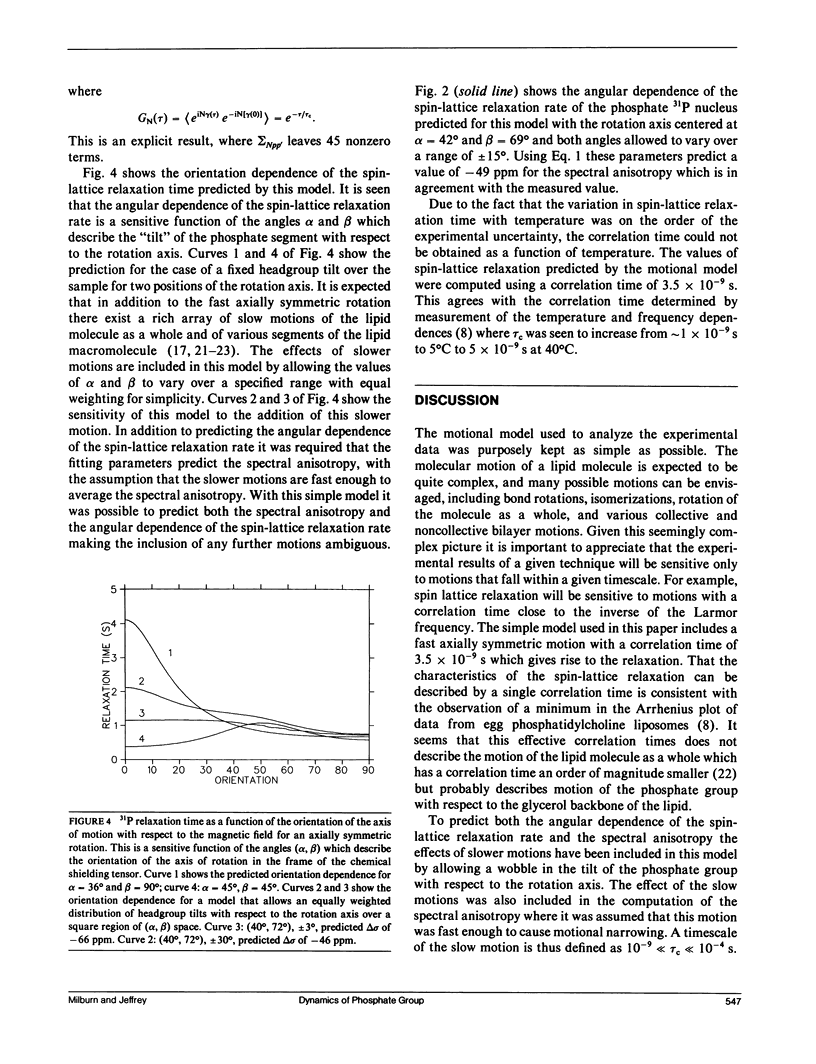
To understand 31P relaxation processes and hence molecular dynamics in the phospholipid multilayer it is important to measure the dependence of the 31P spin-lattice relaxation time on as many variables as the physical system allows. Such measurements of the 31P spin-lattice relaxation rate have been reported both as a function of Larmor frequency and temperature for egg phosphatidylcholine liposomes (Milburn, M.P., and K.R. Jeffrey. 1987. Biophys. J. 52:791-799). In principle, the spin-lattice relaxation rate in an anisotropic environment such as a bilayer will be a function of the angle between the bilayer normal and the magnetic field. However, the measurement of this angular dependence has not been possible because the rapid (on the time-scale of the spin-lattice relaxation rate) diffusion of the lipid molecules over the curved surface of the liposome average this dependence (Milburn, M.P., and K.R. Jeffrey. 1987. Biophys. J. 52:791-799; Brown, M.F., and J.H. Davis. 1981. Chem. Phys. Lett. 79:431-435). This paper reports the results of the measurement of the 31P spin-lattice relaxation rate as a function of this angle, beta', (the angle between the bilayer normal and the external magnetic field) using samples oriented between glass plates. These measurements were made at high field (145.7 MHz) where the spin-lattice relaxation processes are dominated by the chemical shielding interaction (Milburn, M.P., and K.R. Jeffrey. 1987. Biophys. J. 52:791-799). A model of molecular motion that includes a fast axially symmetric rotation of the phosphate group (tau i approximately 10(-9) s) and a wobble of the head group tilt with respect to this rotation axis has been used to describe both the angular dependence of the spin-lattice relaxation and the spectral anisotropy. Cholesterol is seen to have a negligible effect on the motional properties of the phospholipid phosphate segment as measured by the orientation dependence of the spin-lattice relaxation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. F., Seelig J. Influence of cholesterol on the polar region of phosphatidylcholine and phosphatidylethanolamine bilayers. Biochemistry. 1978 Jan 24;17(2):381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. Orientation and dynamics of phospholipid head groups in bilayers and membranes determined from 31P nuclear magnetic resonance chemical shielding tensors. Biochemistry. 1977 Feb 8;16(3):519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- Lange A., Marsh D., Wassmer K. H., Meier P., Kothe G. Electron spin resonance study of phospholipid membranes employing a comprehensive line-shape model. Biochemistry. 1985 Jul 30;24(16):4383–4392. doi: 10.1021/bi00337a020. [DOI] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P nuclear relaxation time study. Biophys J. 1987 Nov;52(5):791–799. doi: 10.1016/S0006-3495(87)83273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderberger W., Seelig J. Phosphorus-31 chemical shift anisotropy in unsonicated phospholipid bilayers. J Am Chem Soc. 1976 Jun 9;98(12):3704–3706. doi: 10.1021/ja00428a053. [DOI] [PubMed] [Google Scholar]
- Peng Z. Y., Simplaceanu V., Lowe I. J., Ho C. Rotating-frame relaxation studies of slow motions in fluorinated phospholipid model membranes. Biophys J. 1988 Jul;54(1):81–95. doi: 10.1016/S0006-3495(88)82933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Kang S. Y., Gutowsky H. S., Oldfield E. Phosphorus nuclear magnetic resonance study of membrane structure. Interactions of lipids with protein, polypeptide, and cholesterol. J Biol Chem. 1981 Feb 10;256(3):1160–1166. [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally G. U., Wohlgemuth R. Orientation and flexibility of the choline head group in phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Jun 2;467(2):109–119. doi: 10.1016/0005-2736(77)90188-2. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally H. Investigation of phosphatidylethanolamine bilayers by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1976 Nov 30;15(24):5199–5204. doi: 10.1021/bi00669a001. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Ruocco M. J., Olejniczak E. T., Das Gupta S. K., Griffin R. G. Anisotropic 2H-nuclear magnetic resonance spin-lattice relaxation in cerebroside- and phospholipid-cholesterol bilayer membranes. Biophys J. 1988 Sep;54(3):373–381. doi: 10.1016/S0006-3495(88)82970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


