Abstract
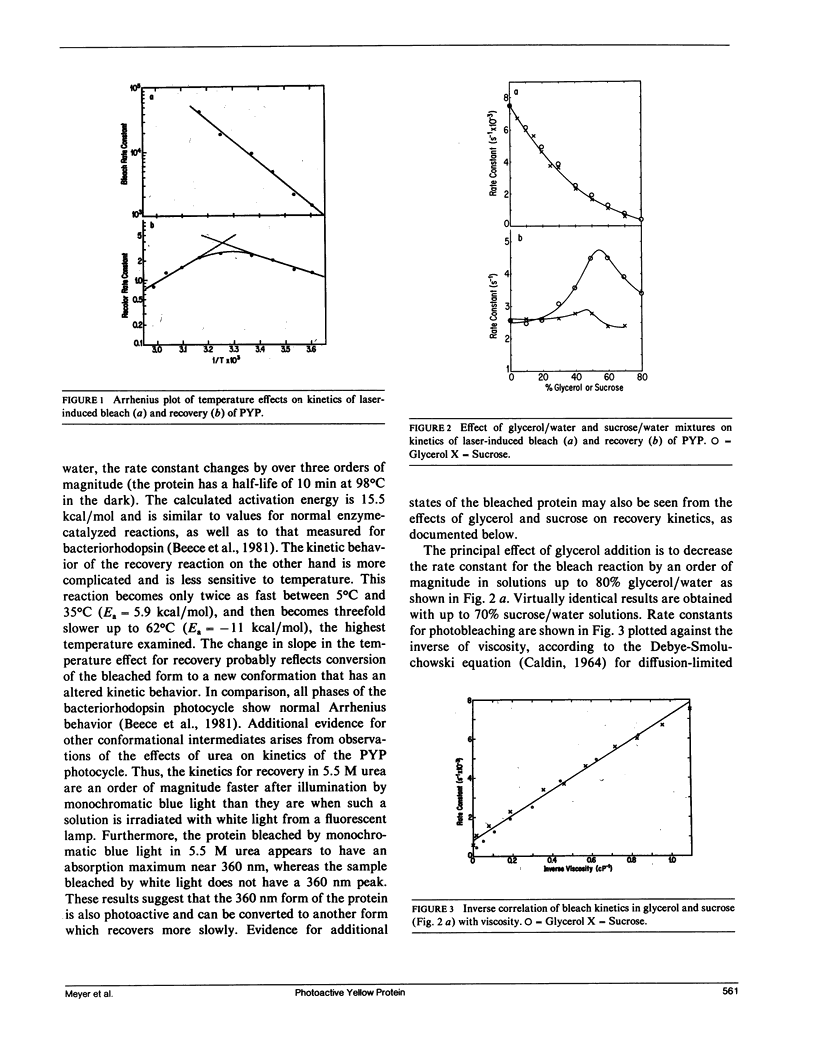
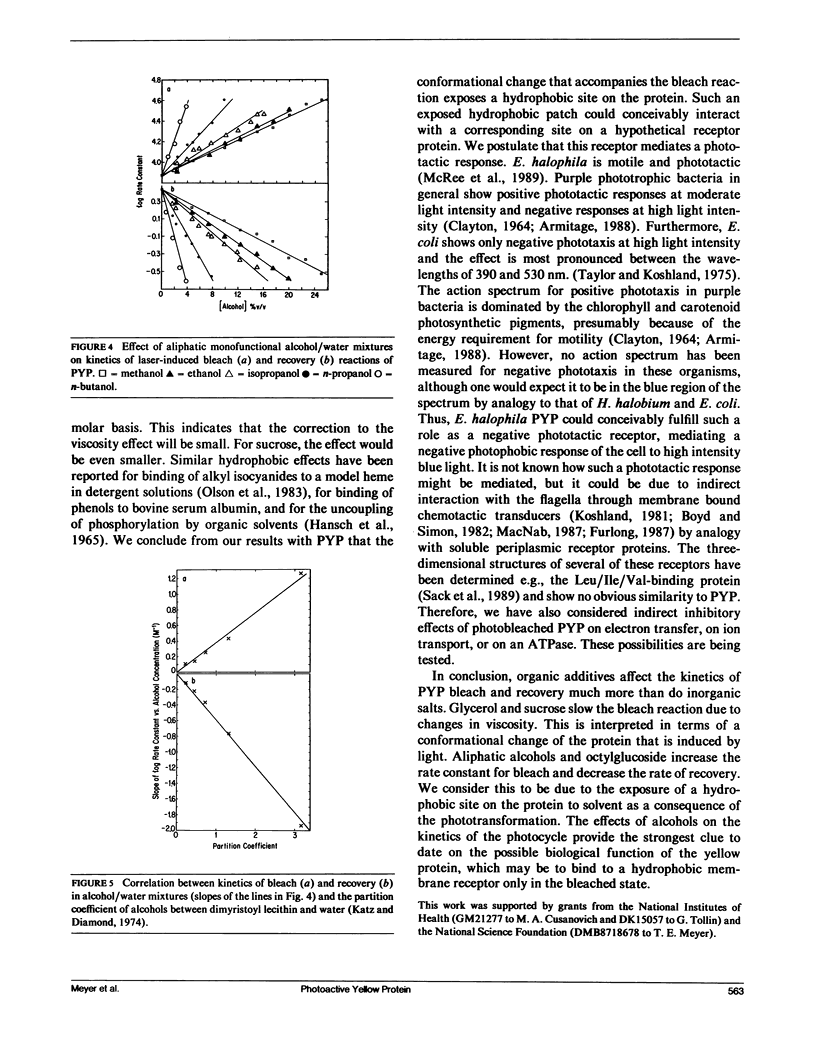
A water-soluble yellow protein from E. halophila was previously shown to be photoactive (Meyer, T. E., E. Yakali, M. A. Cusanovich, and G. Tollin. 1987. Biochemistry. 26:418-423). Pulsed laser excitation in the protein visible absorption band (maximum at 445 nm) causes a rapid bleach of color (k = 7.5 x 10(3) s-1) followed by a slower dark recovery (k = 2.6 s-1). This is analogous to the photocycle of sensory rhodopsin II from Halobacterium (which also has k = 2.6 s-1 for recovery). We have now determined the quantum yield of the photobleaching process to be 0.64, which is comparable with that of bacteriorhodopsin (0.25), and is thus large enough to be biologically significant. Although the photoreactions of yellow protein were previously shown to be relatively insensitive to pH, ionic strength and the osmoregulator betaine, the present experiments demonstrate that temperature, glycerol, sucrose, and various alcohol-water mixtures strongly influence the kinetics of photobleaching and recovery. The effect of temperature follows normal Arrhenius behavior for the bleach reaction (Ea = 15.5 kcal/mol). The rate constant for the recovery reaction increases with temperature between 5 degrees C and 35 degrees C, but decreases above 35 degrees C indicating alternate conformations with differing kinetics. There is an order of magnitude decrease in the rate constant for photobleaching in both glycerol and sucrose solutions that can be correlated with the changes in viscosity. We conclude from this that the protein undergoes a conformational change as a consequence of the photoinduced bleach. Recovery kinetics are affected by glycerol and sucrose to a much smaller extent and in a more complicated manner. Aliphatic, monofunctional alcohol-water solutions increase the rate constant for the bleach reaction and decrease the rate constant for the recovery reaction, each by an order of magnitude. These effects do not correlate with dielectric constant, indicating that the photocycle probably does not involve separation or recombination of charge accessible to the protein surface. However, the effects on both bleaching and recovery correlate well with the relative hydrophobicity(as measured by partition coefficients in detergent/water mixtures), in the order of increasing effectiveness:methanol < ethanol < iso-propanol <n-propanol < n-butanol. We conclude that the change in conformation of the protein induced by light exposes a hydrophobic site to the solvent. This suggests the possibility that light exerts its effect in vivo by exposing a region of the protein for binding to a hydrophobic receptor site in the cell, perhaps to a protein analogous to the chemotactic transducers in the cytoplasmic membranes of enteric bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Varani G. The role of the solvent on the binding of ethidium bromide to DNA in alcohol-water mixtures. Biopolymers. 1986 Nov;25(11):2187–2208. doi: 10.1002/bip.360251111. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Hansch C., Kiehs K., Lawrence G. L. The role of substituents in the hydrophobic bonding of phenols by serum and mitochondrial proteins. J Am Chem Soc. 1965 Dec 20;87(24):5770–5773. doi: 10.1021/ja00952a044. [DOI] [PubMed] [Google Scholar]
- Katz Y., Diamond J. M. Thermodynamic constants for nonelectrolyte partition between dimyristoyl lecithin and water. J Membr Biol. 1974;17(2):101–120. doi: 10.1007/BF01870175. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- McRee D. E., Meyer T. E., Cusanovich M. A., Parge H. E., Getzoff E. D. Crystallographic characterization of a photoactive yellow protein with photochemistry similar to sensory rhodopsin. J Biol Chem. 1986 Oct 15;261(29):13850–13851. [PubMed] [Google Scholar]
- Meyer T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim Biophys Acta. 1985 Jan 23;806(1):175–183. doi: 10.1016/0005-2728(85)90094-5. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Yakali E., Cusanovich M. A., Tollin G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry. 1987 Jan 27;26(2):418–423. doi: 10.1021/bi00376a012. [DOI] [PubMed] [Google Scholar]
- Rayfield G. W. Evidence that charge motion within bacteriorhodopsin depends on solvent viscosity. Photochem Photobiol. 1986 Feb;43(2):171–174. doi: 10.1111/j.1751-1097.1986.tb09510.x. [DOI] [PubMed] [Google Scholar]
- Sack J. S., Saper M. A., Quiocho F. A. Periplasmic binding protein structure and function. Refined X-ray structures of the leucine/isoleucine/valine-binding protein and its complex with leucine. J Mol Biol. 1989 Mar 5;206(1):171–191. doi: 10.1016/0022-2836(89)90531-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Intrinsic and extrinsic light responses of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1975 Aug;123(2):557–569. doi: 10.1128/jb.123.2.557-569.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


