Abstract
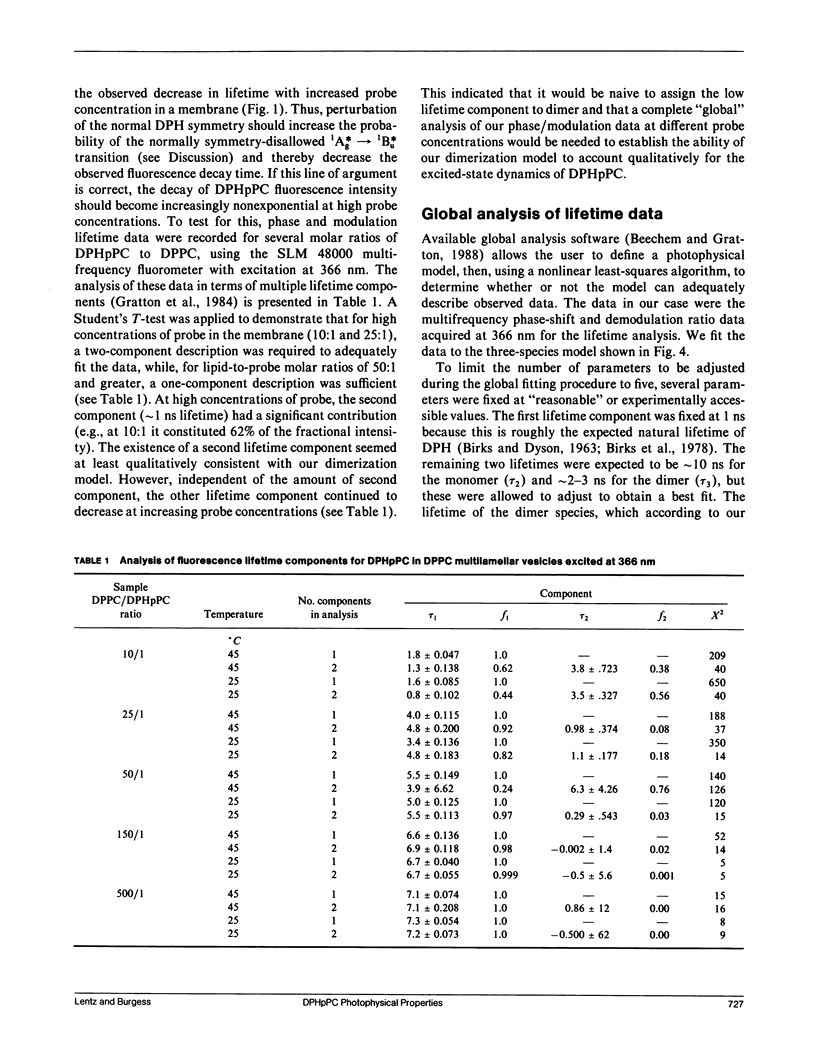
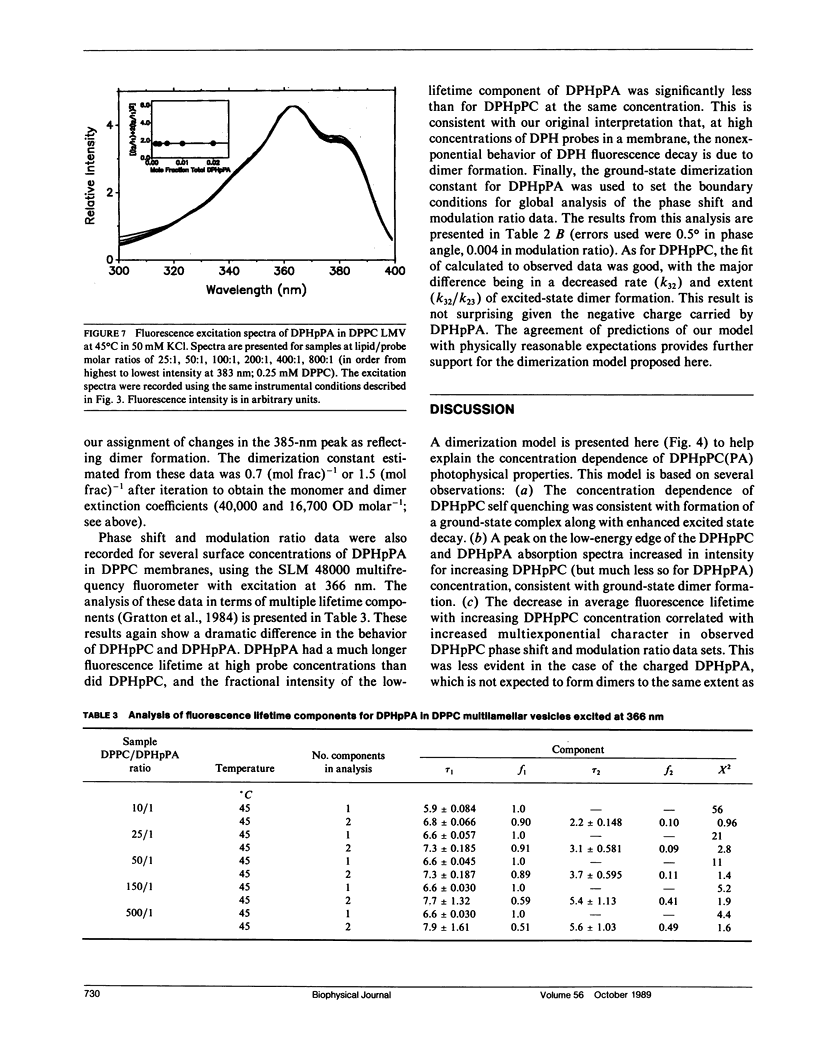
We have investigated the reason for the sensitivity of the fluorescence excited-state lifetime of 1,6-diphenyl-1,3,5-hexatriene (DPH) and its phospholipid derivatives, 1-palmitoyl-2-[2-[4-(6-phenyl-trans-1,3,5- hexatrienyl)phenyl]ethyl)carbonyl)-3-sn-phosphatidylcholine (DPHpPC) and 1-palmitoyl-2-[2-[4-(6-phenyl-trans-1,3,5- hexatrienyl)phenyl]ethyl)carbonyl)-3-sn-phosphatidic acid (DPHpPA), to the concentration of these probes in dipalmitoylphosphatidylcholine (DPPC) multilamellar membranes (Barrow, D. A., and B. R. Lentz, 1985. Biophys. J. 48:221-234; Parente, R. A., and B. R. Lentz. 1985. Biochemistry. 24:6178-6185). We have interpreted self-quenching data, excitation and emission spectra, and phase and modulation lifetime data in terms of a model that envisions dimerization of these probes in a membrane bilayer. It is proposed that dimerization alters the symmetry of the DPH excited state so as to allow more rapid decay via the normally symmetry-disallowed route from the 1Ag* state. Global analysis of fluorescence phase shift and modulation ratio data for DPHpPC in terms of the dimerization model provided a good fit of these data as a function of both modulation frequency and probe concentration. Global analysis of a similar set of data for the charged phosphatide DPHpPA predicted that this probe was much less prone to dimerize than was the uncharged DPHpPC. This physically reasonable result provides support for the assumptions made in the development of our model. We conclude that the dimerization model allows rationalization of many of the anomalous photophysical properties of DPH and its derivatives in membranes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow D. A., Lentz B. R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys J. 1985 Aug;48(2):221–234. doi: 10.1016/S0006-3495(85)83775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow D. A., Lentz B. R. The use of isochronal reference standards in phase and modulation fluorescence lifetime measurements. J Biochem Biophys Methods. 1983 May;7(3):217–234. doi: 10.1016/0165-022x(83)90031-3. [DOI] [PubMed] [Google Scholar]
- Chen L. A., Dale R. E., Roth S., Brand L. Nanosecond time-dependent fluorescence depolarization of diphenylhexatriene in dimyristoyllecithin vesicles and the determination of "microviscosity". J Biol Chem. 1977 Apr 10;252(7):2163–2169. [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Davenport L., Dale R. E., Bisby R. H., Cundall R. B. Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985 Jul 16;24(15):4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- Duportail G., Weinreb A. Photochemical changes of fluorescent probes in membranes and their effect on the observed fluorescence anisotropy values. Biochim Biophys Acta. 1983 Dec 21;736(2):171–177. doi: 10.1016/0005-2736(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Gratton E., Limkeman M., Lakowicz J. R., Maliwal B. P., Cherek H., Laczko G. Resolution of mixtures of fluorophores using variable-frequency phase and modulation data. Biophys J. 1984 Oct;46(4):479–486. doi: 10.1016/S0006-3495(84)84044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iioka H., Moriyama I., Akazaki M., Oku M., Itoh K., Hino K., Okamura Y., Itani Y., Katoh Y., Ichijo M. [The study on human placental DHA-S transport mechanism (using placental microvillous membrane vesicles)]. Nihon Sanka Fujinka Gakkai Zasshi. 1987 Oct;39(10):1756–1760. [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Morgan C. G., Thomas E. W., Moras T. S., Yianni Y. P. The use of a phospholipid analogue of diphenyl-1,3,5-hexatriene to study melittin-induced fusion of small unilamellar phospholipid vesicles. Biochim Biophys Acta. 1982 Nov 8;692(2):196–201. doi: 10.1016/0005-2736(82)90521-1. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Conti F., Glaser M., Gratton E. Detection of phospholipid phase separation. A multifrequency phase fluorimetry study of 1,6-diphenyl-1,3,5-hexatriene fluorescence. J Biol Chem. 1984 Nov 25;259(22):14011–14017. [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Advantages and limitations of 1-palmitoyl-2-[[2-[4- (6-phenyl-trans-1,3,5-hexatrienyl)phenyl]ethyl]carbonyl]-3- sn-phosphatidylcholine as a fluorescent membrane probe. Biochemistry. 1985 Oct 22;24(22):6178–6185. doi: 10.1021/bi00343a022. [DOI] [PubMed] [Google Scholar]
- Parente R. A., Lentz B. R. Fusion and phase separation monitored by lifetime changes of a fluorescent phospholipid probe. Biochemistry. 1986 Mar 11;25(5):1021–1026. doi: 10.1021/bi00353a011. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Haugland R. P., Callahan P. J. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: synthesis, fluorescence properties, and use as a fluorescence probe of lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]


