Abstract
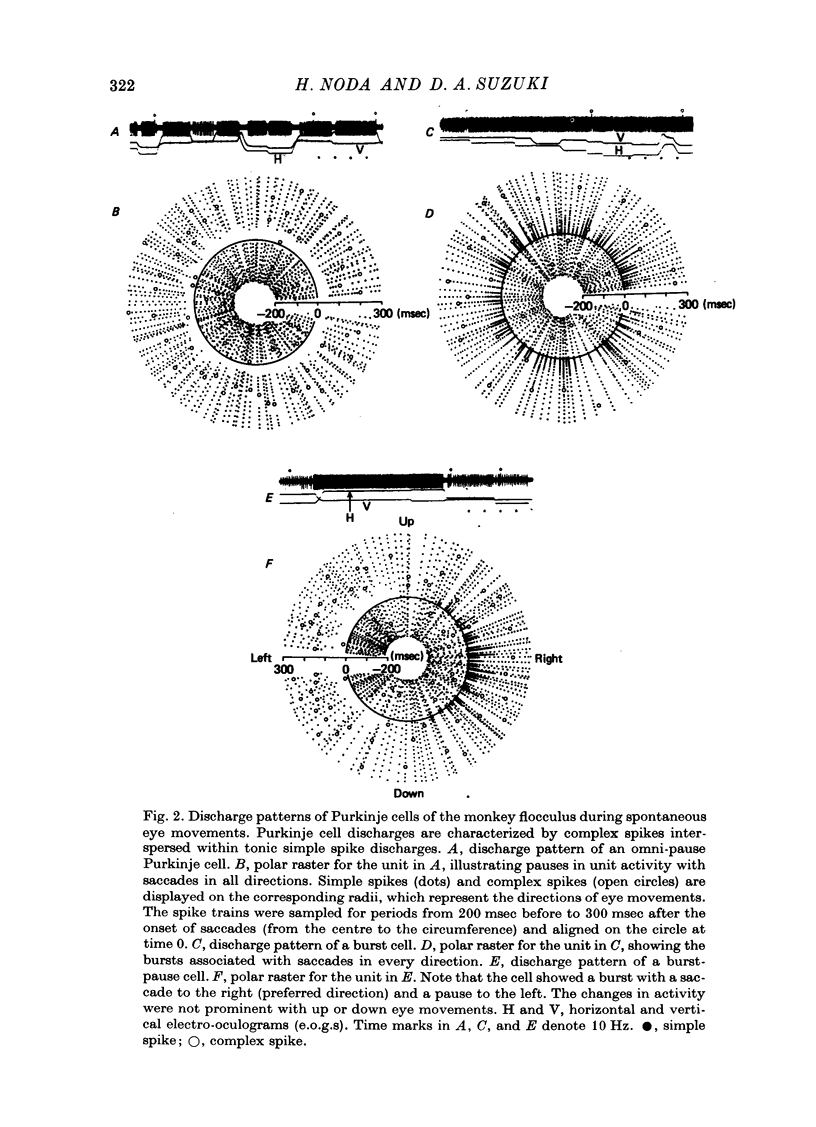
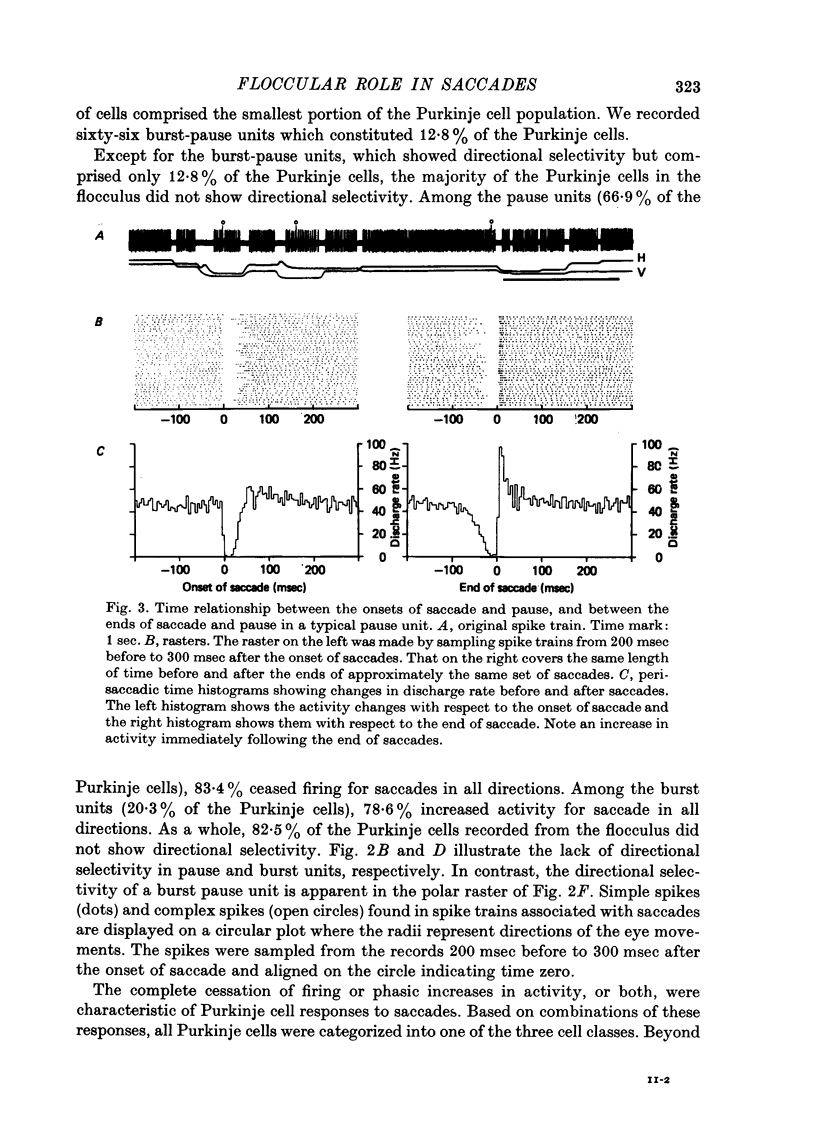
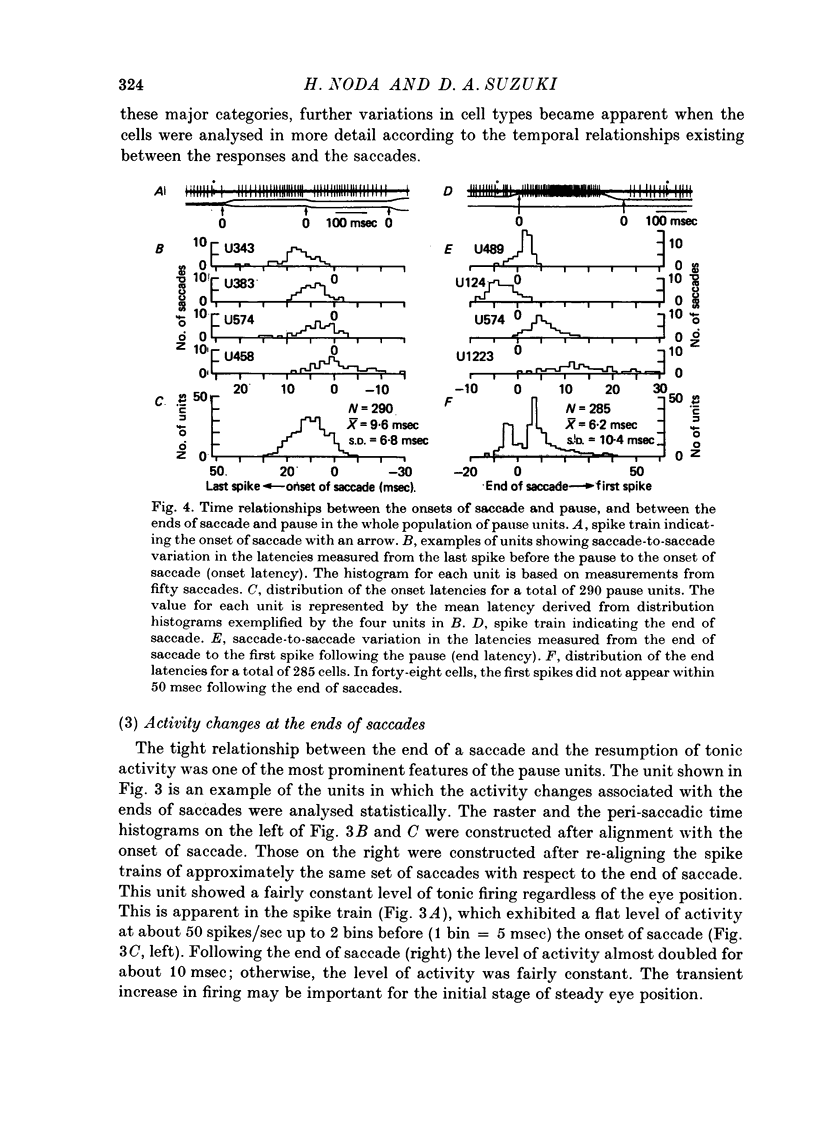
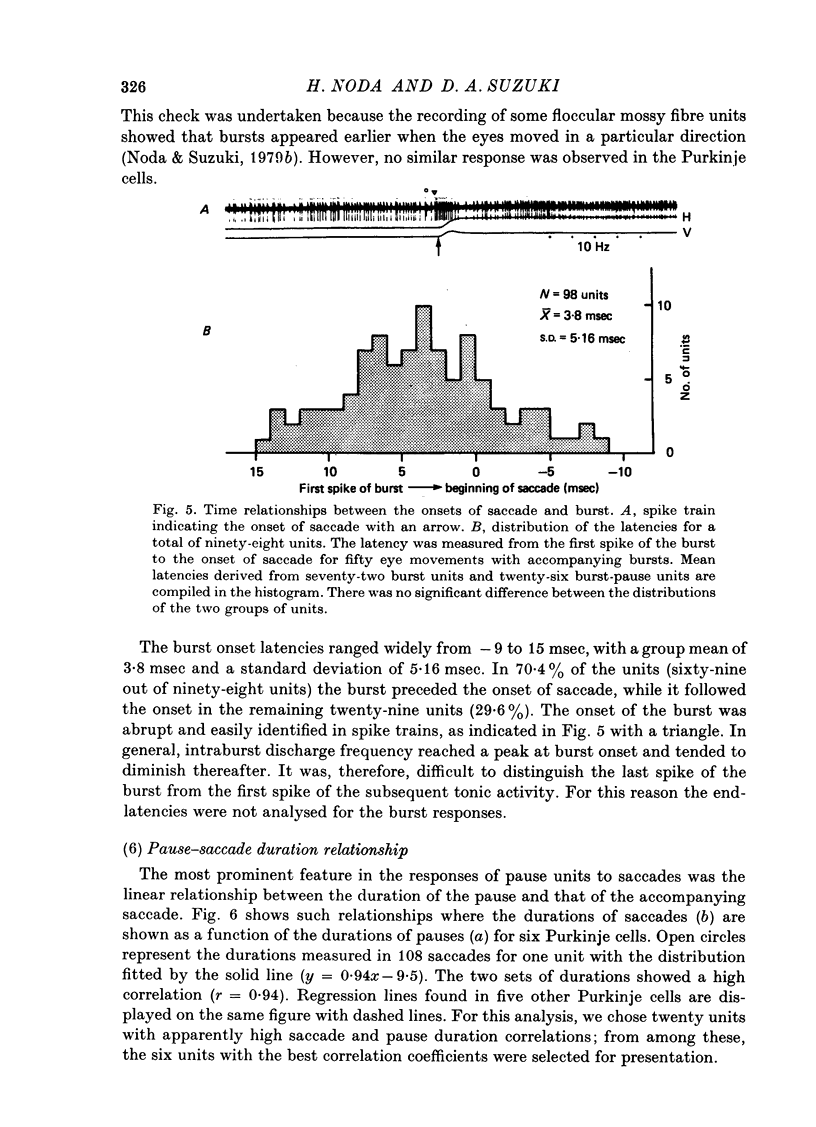
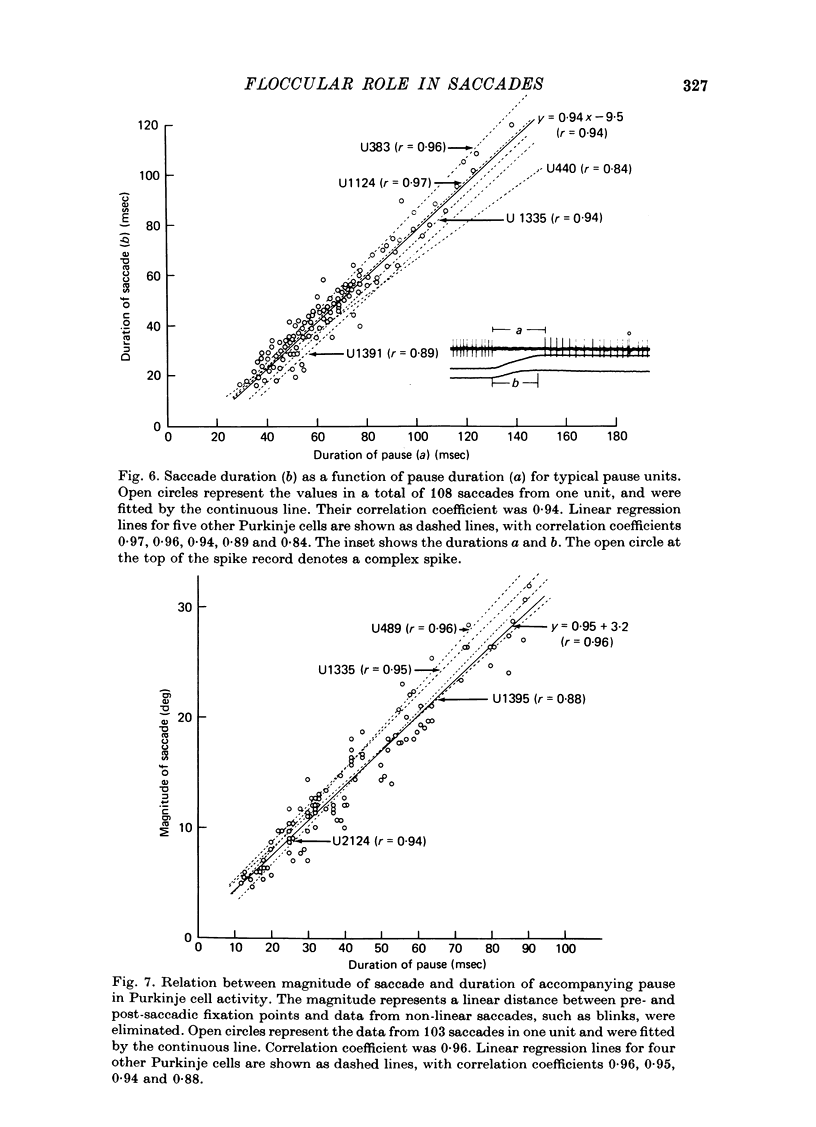
1. Purkinje cell discharges were recorded from the flocculus of monkeys either spontaneously making saccadic eye movements (saccades) or trained to fixate a small visual target presented on a tangent screen. In the trained monkeys, saccades of known magnitude and direction were induced by changing the position of the fixation target. 2. Among 513 Purkinje cells, 343 units (66.9%) paused during saccades in all directions (286 units) or in particular directions (57 units). In most units, there were intimate temporal relationships between the beginnings of pauses and saccades, and between the ends of pasuses and saccades. 3. The pause in activity preceded saccades by an average of 9.6 msec, with a maximum lead time of 30 msec. In a fraction of the units (7.6%), the pause started after the onset of saccades. 4. There were 104 units (20.3%) which showed bursts during saccades in all directions (eighty-two units) or in particular directions (twenty-two units). 5. In sixty-six units (12.8%) a burst was associated with saccades in one direction and a pause in the opposite direction. 6. The burst in the burst and burst-pause units preceded saccades by an average of 3.8 msec. There was no significant difference in the lead times between these two groups of units. 7. There was a linear relationship between the duration of the pause in Purkinje cell activity and that of the accompanying saccade. A linear relationship was also seen between the pause duration and the magnitude of saccade.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut P., Brodal A. The projection of the "vestibulocerebellum" onto the vestibular nuclei in the cat. Arch Ital Biol. 1967 Nov;105(4):441–479. [PubMed] [Google Scholar]
- Baker R., Berthoz A., Delgado-García J. Monosynaptic excitation of trochlear motoneurons following electrical stimulation of the prepositus hypoglossi nucleus. Brain Res. 1977 Jan 31;121(1):157–161. doi: 10.1016/0006-8993(77)90445-0. [DOI] [PubMed] [Google Scholar]
- CARPENTER M. B., McMASTERS R. E. Disturbances of conjugate horizontal eye movements in the monkey. II. Physiological effects and anatomical degeneration resulting from lesions in the medical longitudinal fasciculus. Arch Neurol. 1963 Apr;8:347–368. doi: 10.1001/archneur.1963.00460040017001. [DOI] [PubMed] [Google Scholar]
- CARPENTER M. B., STROMINGER N. L. CEREBELLO-OCULOMOTOR FIBERS IN THE RHESUS MONKEY. J Comp Neurol. 1964 Oct;123:211–229. doi: 10.1002/cne.901230206. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Strominger N. L. The medial longitudinal fasciculus and disturbances of conjugate horizontal eye movements in the monkey. J Comp Neurol. 1965 Aug;125(1):41–65. doi: 10.1002/cne.901250106. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. F., Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol. 1975 Sep;38(5):1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner E. P., Fuchs A. F. Single-unit responses to natural vestibular stimuli and eye movements in deep cerebellar nuclei of the alert rhesus monkey. J Neurophysiol. 1975 May;38(3):627–649. doi: 10.1152/jn.1975.38.3.627. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Hartwieg E. A. Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res. 1974 Dec 13;81(3):543–551. doi: 10.1016/0006-8993(74)90850-6. [DOI] [PubMed] [Google Scholar]
- Haines D. E. Cerebellar corticonuclear and corticovestibular fibers of the flocculonodular lobe in a prosimian primate (Galago senegalensis). J Comp Neurol. 1977 Aug 15;174(4):607–630. doi: 10.1002/cne.901740405. [DOI] [PubMed] [Google Scholar]
- Henn V., Cohen B. Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res. 1976 May 28;108(2):307–325. doi: 10.1016/0006-8993(76)90188-8. [DOI] [PubMed] [Google Scholar]
- Henn V., Cohen B. Quantitative analysis of activity in eye muscle motoneurons during saccadic eye movements and positions of fixation. J Neurophysiol. 1973 Jan;36(1):115–126. doi: 10.1152/jn.1973.36.1.115. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Kamath B. Y. Characteristics of head rotation and eye movement-related neurons in alert monkey vestibular nucleus. Brain Res. 1975 Dec 12;100(1):182–187. doi: 10.1016/0006-8993(75)90257-7. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Kotchabhakdi N., Walberg F. Cerebeller afferents from neurons in motor nuclei of cranial nerves demonstrated by retrograde axonal transport of horseradish peroxidase. Brain Res. 1977 Nov 25;137(1):158–163. doi: 10.1016/0006-8993(77)91020-4. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978 May;41(3):733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Luschei E. S., Fuchs A. F. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972 Jul;35(4):445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. J., Kaneko C. R., Highstein S. M., Eagen K. Vestibular and medullary brain stem afferents to the abducens nucleus in the cat. Brain Res. 1977 Mar 11;123(2):229–240. doi: 10.1016/0006-8993(77)90476-0. [DOI] [PubMed] [Google Scholar]
- Mano N. Changes of simple and complex spike activity of cerebellar purkinje cells with sleep and waking. Science. 1970 Dec 18;170(3964):1325–1327. doi: 10.1126/science.170.3964.1325. [DOI] [PubMed] [Google Scholar]
- McMasters R. E., Weiss A. H., Carpenter M. B. Vestibular projections to the nuclei of the extraocular muscles. Degeneration resulting from discrete partial lesions of the vestibular nuclei in the monkey. Am J Anat. 1966 Jan;118(1):163–194. doi: 10.1002/aja.1001180109. [DOI] [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H. Visual tracking and the primate flocculus. Science. 1975 Sep 19;189(4207):1000–1002. doi: 10.1126/science.1083068. [DOI] [PubMed] [Google Scholar]
- Miles F. A. Single unit firing patterns in the vestibular nuclei related to voluntary eye movements and passive body rotation in conscious monkeys. Brain Res. 1974 May 17;71(2-3):215–224. doi: 10.1016/0006-8993(74)90963-9. [DOI] [PubMed] [Google Scholar]
- Miles F. A. Single unit firing patterns in the vestibular nuclei related to voluntary eye movements and passive body rotation in conscious monkeys. Brain Res. 1974 May 17;71(2-3):215–224. doi: 10.1016/0006-8993(74)90963-9. [DOI] [PubMed] [Google Scholar]
- Noda H. Depression in the excitability of relay cells of lateral geniculate nucleus following saccadic eye movements in the cat. J Physiol. 1975 Jul;249(1):87–102. doi: 10.1113/jphysiol.1975.sp011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H., Suzuki D. A. Processing of eye movement signals in the flocculus of the monkey. J Physiol. 1979 Sep;294:349–364. doi: 10.1113/jphysiol.1979.sp012934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H., Suzuki D. A. The role of the flocculus of the monkey in fixation and smooth pursuit eye movements. J Physiol. 1979 Sep;294:335–348. doi: 10.1113/jphysiol.1979.sp012933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. W. An anatomical and experimental study of the cerebellar nuclei and their efferent pathways in the monkey. J Comp Neurol. 1954 Aug;101(1):167–223. doi: 10.1002/cne.901010107. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. A. THE MECHANICS OF HUMAN SACCADIC EYE MOVEMENT. J Physiol. 1964 Nov;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. A. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970 May;33(3):393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Ron S., Robinson D. A. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973 Nov;36(6):1004–1022. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schlag J., Lehtinen I., Schlag-Rey M. Neuronal activity before and during eye movements in thalamic internal medullary lamina of the cat. J Neurophysiol. 1974 Sep;37(5):982–995. doi: 10.1152/jn.1974.37.5.982. [DOI] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974 Jun 7;72(2):213–224. doi: 10.1016/0006-8993(74)90860-9. [DOI] [PubMed] [Google Scholar]
- Tarlov E. Organization of vestibulo-oculomotor projections in the cat. Brain Res. 1970 Jun 3;20(2):159–179. doi: 10.1016/0006-8993(70)90286-6. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970 Jul;33(4):537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Oculomotor defects in cerebellectomized monkeys. Invest Ophthalmol. 1973 Aug;12(8):618–621. [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J Neurophysiol. 1972 Jul;35(4):575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]


