Abstract
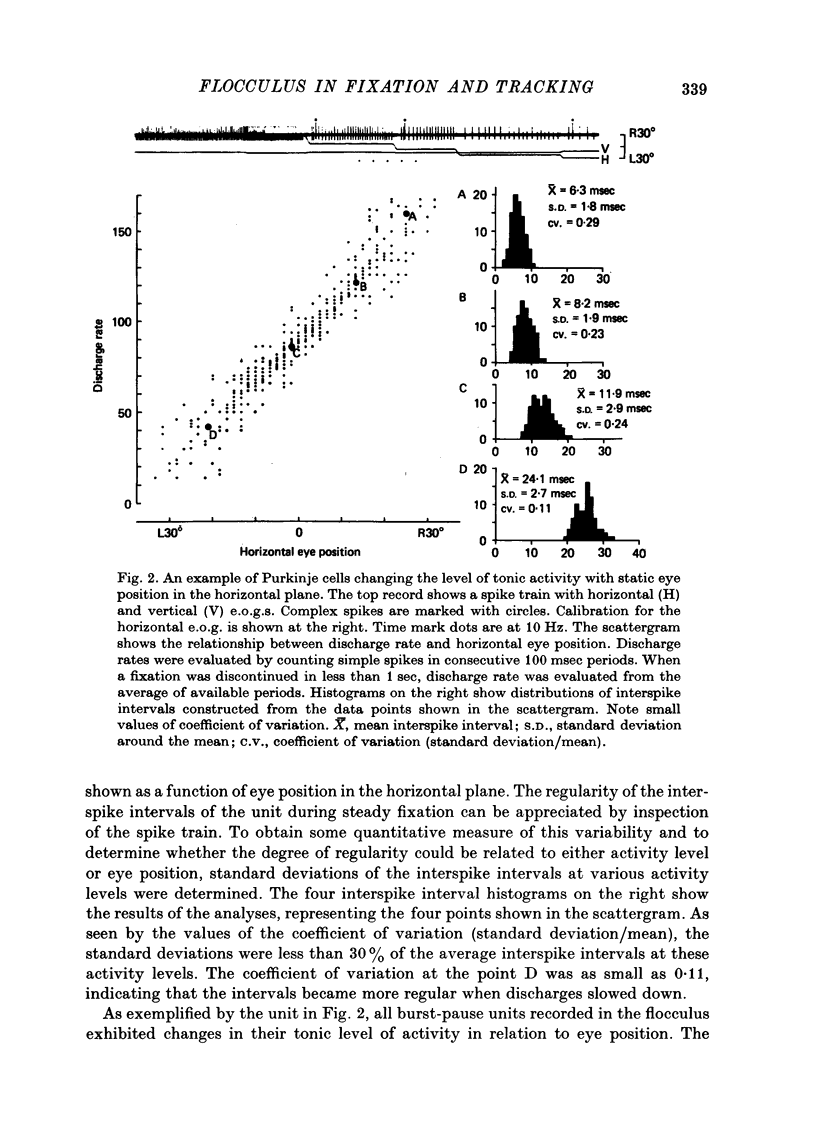
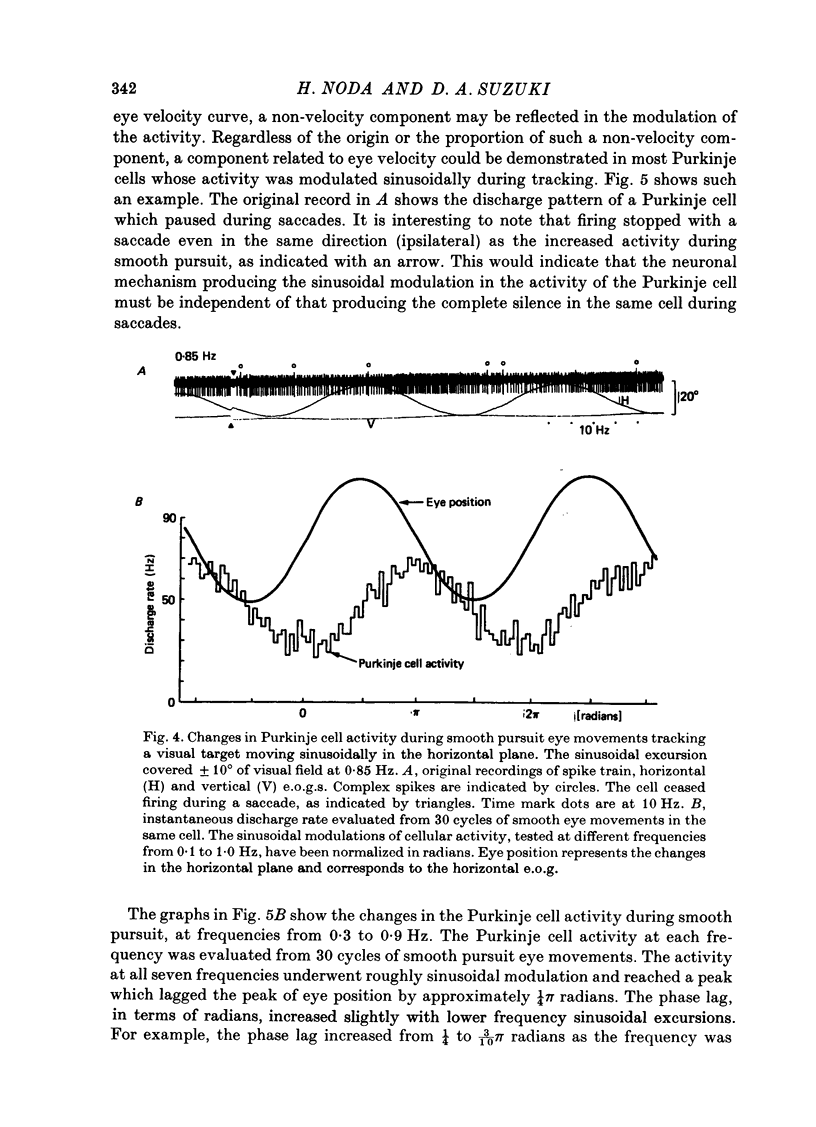
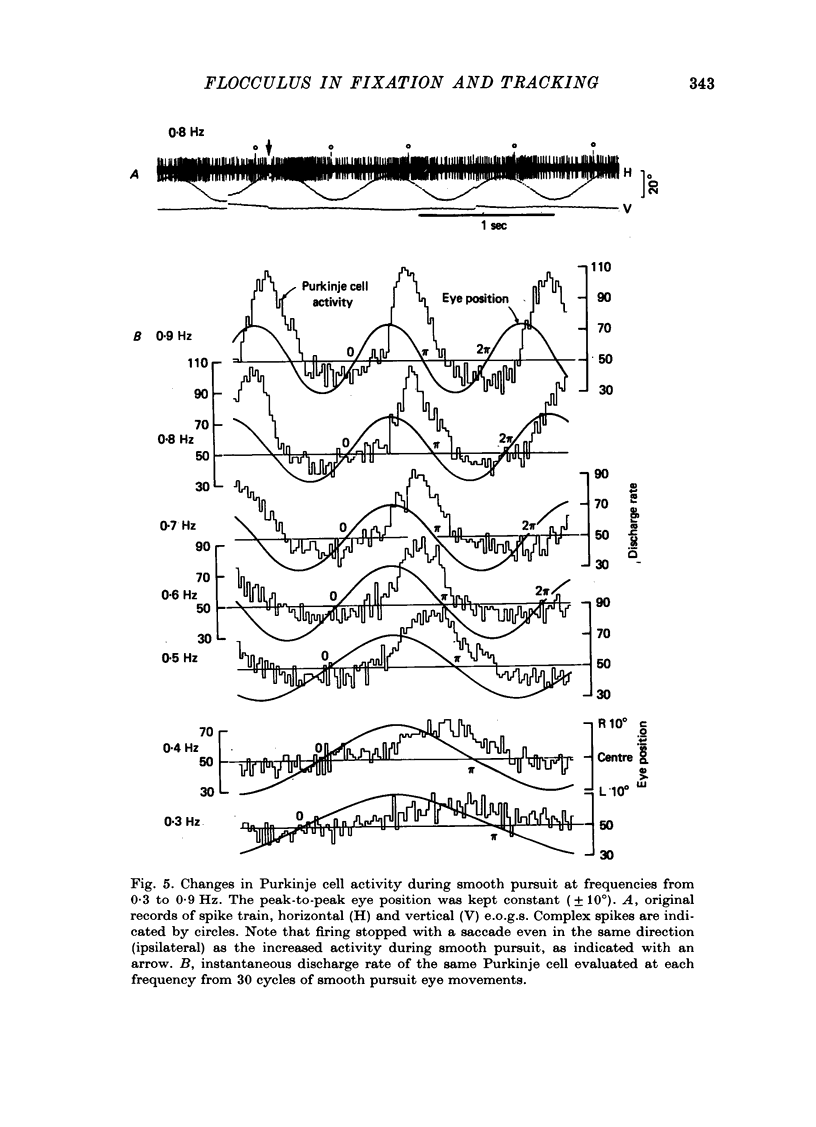
1. Purkinje cell discharges were recorded from the flocculus of monkeys trained to fixate a small visual target and to track the target when moved slowly. 2. A striking feature of Purkinje cell activity during the steady fixation was a high rate of tonic discharges with regular interspike intervals. The average discharge rate in the whole population of Purkinje cells ranged from 37 to 145 spikes/sec. The coefficient of variation of the interspike intervals was typically smaller than 0.5 in most units. 3. In 43.9% of the Purkinje cells, tonic levels of activity changed by more than 20% of the average background activity with shifts of gaze. 4. In some Purkinje cells, especially in most burst-pause units, discharge rates during steady fixation were proportional to eye positions in one plane, implicating these cells as sources of eye position information to their target cells. 5. When the monkey tracked a sinusoidally oscillating target, the activity of some Purkinje cells was clearly modulated in phase with the eye velocity. 6. In the other Purkinje cells exhibiting smooth pursuit modulation, the activity curve appeared with a phase shift. When these cells were tested with sinusoidal target movements at different frequencies, but with a constant magnitude, the peak firing rates were proportional to the frequencies of the excursions, i.e. the velocities. 7. The flocculus thus provides the oculomotor system with eye position information during fixation and with velocity information during smooth pursuit and participates in the control of oculomotor functions stabilizing retinal images.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FISHER C. M., PICARD E. H., POLAK A., DALAL P., POJEMANN R. G. ACUTE HYPERTENSIVE CEREBELLAR HEMORRHAGE: DIAGNOSIS AND SURGICAL TREATMENT. J Nerv Ment Dis. 1965 Jan;140:38–57. doi: 10.1097/00005053-196501000-00004. [DOI] [PubMed] [Google Scholar]
- Kornhuber H. H. Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetik. 1971 Apr;8(4):157–162. doi: 10.1007/BF00290561. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978 May;41(3):733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H. Visual tracking and the primate flocculus. Science. 1975 Sep 19;189(4207):1000–1002. doi: 10.1126/science.1083068. [DOI] [PubMed] [Google Scholar]
- Miles F. A. Single unit firing patterns in the vestibular nuclei related to voluntary eye movements and passive body rotation in conscious monkeys. Brain Res. 1974 May 17;71(2-3):215–224. doi: 10.1016/0006-8993(74)90963-9. [DOI] [PubMed] [Google Scholar]
- Nashold B. S., Jr, Slaughter D. G. Effects of stimulating or destroying the deep cerebellar regions in man. J Neurosurg. 1969 Aug;31(2):172–186. doi: 10.3171/jns.1969.31.2.0172. [DOI] [PubMed] [Google Scholar]
- Noda H., Suzuki D. A. The role of the flocculus of the monkey in saccadic eye movements. J Physiol. 1979 Sep;294:317–334. doi: 10.1113/jphysiol.1979.sp012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol. 1976 Nov;39(6):1246–1256. doi: 10.1152/jn.1976.39.6.1246. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970 May;33(3):393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974 Jun 7;72(2):213–224. doi: 10.1016/0006-8993(74)90860-9. [DOI] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Function Organization of primate oculomotor system revealed by cerebellectomy. Exp Brain Res. 1974;21(5):463–472. doi: 10.1007/BF00237165. [DOI] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Oculomotor defects in cerebellectomized monkeys. Invest Ophthalmol. 1973 Aug;12(8):618–621. [PubMed] [Google Scholar]
- Zee D. S., Optican L. M., Cook J. D., Robinson D. A., Engel W. K. Slow saccades in spinocerebellar degeneration. Arch Neurol. 1976 Apr;33(4):243–251. doi: 10.1001/archneur.1976.00500040027004. [DOI] [PubMed] [Google Scholar]


