Abstract
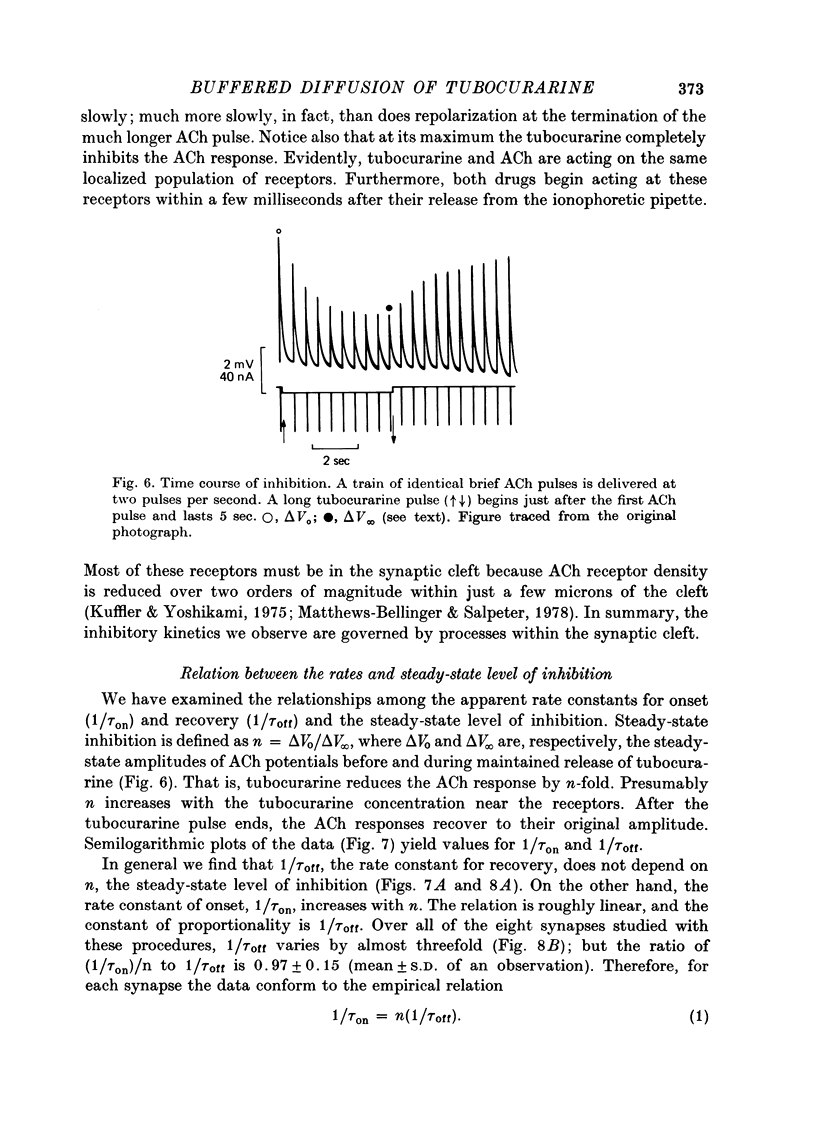
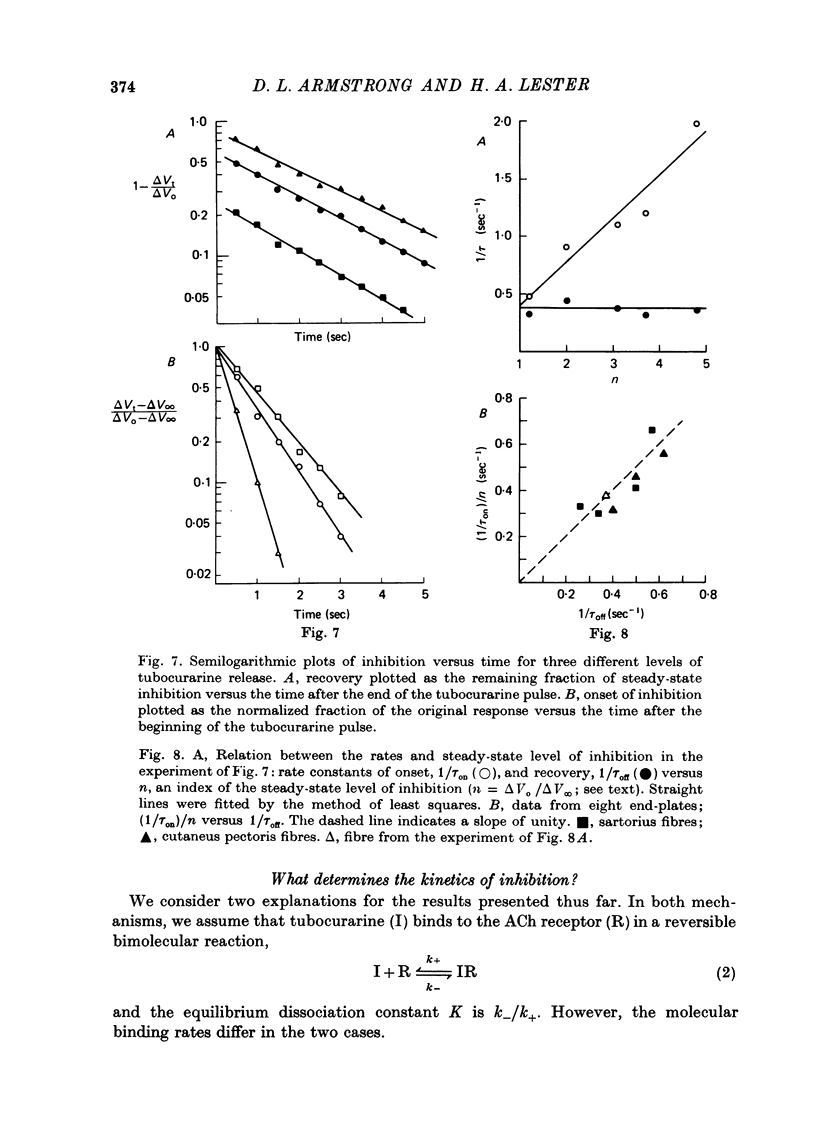
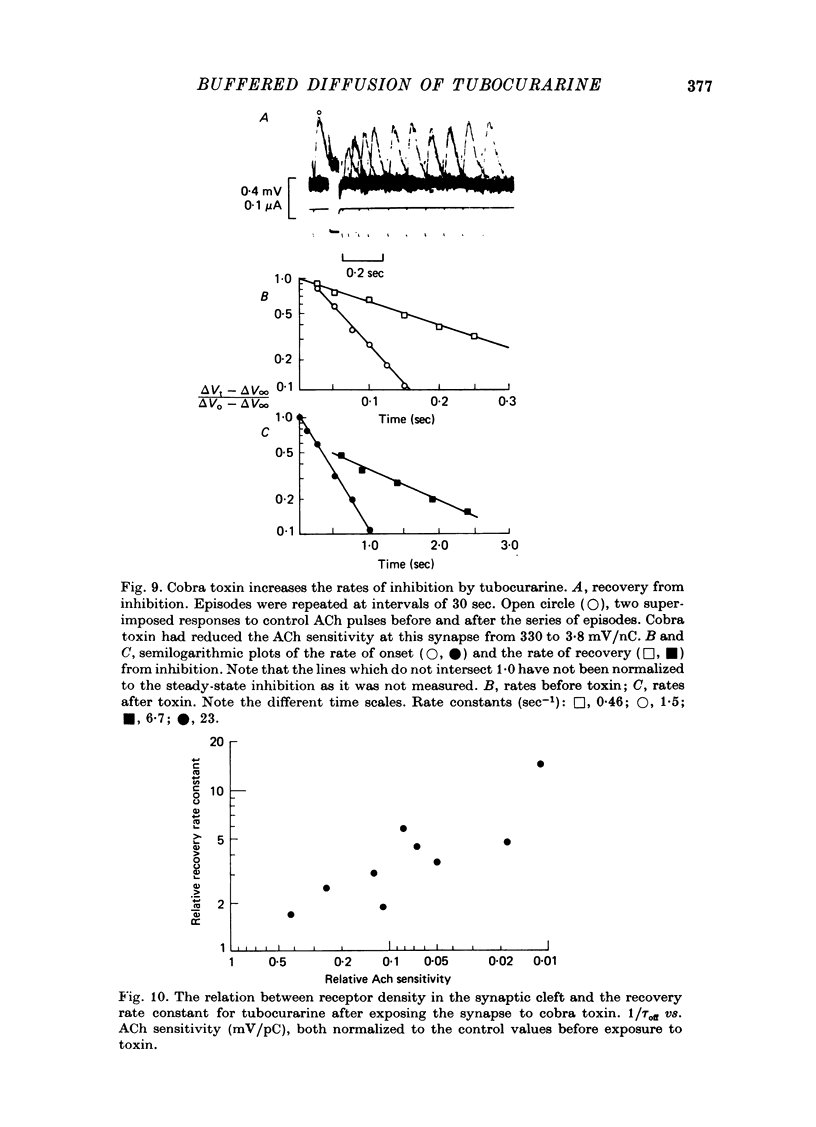
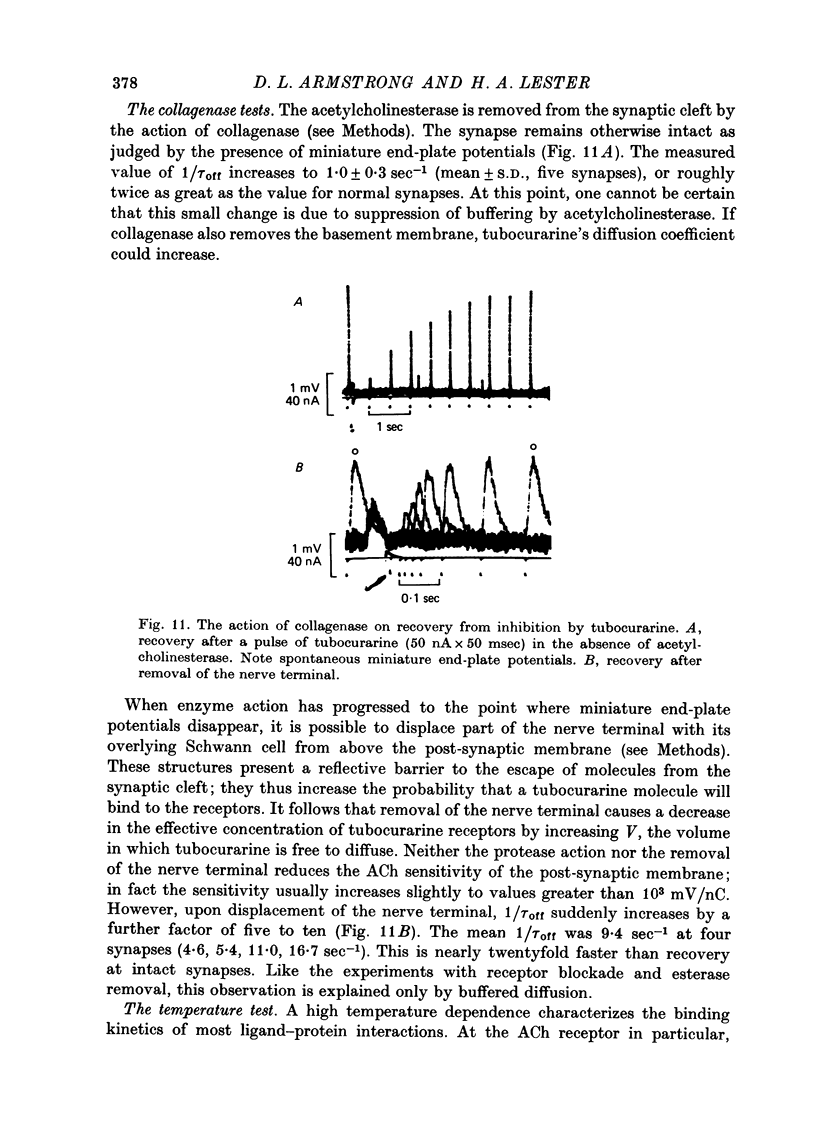
1. The kinetics of tubocurarine inhibition were studied at the post-synaptic membrane of frog skeletal muscle fibres. Acetylcholine (ACh) and (+)-tubocurarine were ionophoresed from twin-barrel micropipettes, and the membrane potential of the muscle fibre was recorded intracellularly. Tubocurarine-receptor binding was measured by decreases in the response to identical pulses of ACh. 2. The responses to both ACh and tubocurarine had brief latencies and reached their maxima rapidly. It is suggested that under these conditions the kinetics of tubocurarine action are not slowed by diffusion in the space outside the synaptic cleft. 3. After a pulse of tubocurarine, recovery from inhibition proceeds along a roughly exponential time course with a rate constant, 1/tau off approximately equal to 0.5 sec-1. This rate constant does not depend on the maximal level of inhibition and varies only slightly with temperature (Q10 = 1.25). 4. After a sudden maintained increase in tubocurarine release, the ACh responses decrease and eventually reach a new steady-state level. Inhibition develops exponentially with time and the apparent rate constant, 1/tau on, is greater than 1/tau off. When the steady-state inhibition reduces the ACh response to 1/n of its original level, the data are summarized by the relation, 1/tau on = n(1/tau off). 5. When the ACh sensitivity is reduced with cobra toxin, both 1/tau on and 1/tau off increase. Thus, the kinetics of tubocurarine inhibition depend on the density of ACh receptors in the synaptic cleft. 6. After treatment with collagenase, part of the nerve terminal is displaced and the post-synaptic membrane is exposed directly to the external solution. Under these circumstances, 1/tau off increases more than tenfold. 7. Bath-applied tubocurarine competitively inhibits the responses to brief ionophoretic ACh pulses with an apparent equilibrium dissociation constant, K = 0.5 microM. 8. In denervated frog muscle fibres, extrasynaptic receptors have a lower apparent affinity for tubocurarine. After a pulse of tubocurarine, inhibition decays tenfold more rapidly at these extrasynaptic sites than at the synapse. 9. It is suggested that each tubocurarine molecule binds repeatedly to several ACh receptors before escaping from the synaptic from the synaptic cleft and that the probability of this repetitive binding is enhanced because the nerve terminal presents a physical barrier to diffusion out of the cleft. Consequently, the receptor transiently buffer the concentration of tubocurarine in the cleft, and the macroscopic kinetics of inhibition are much slower than the molecular binding rates for tubocurarine.
Full text
PDF


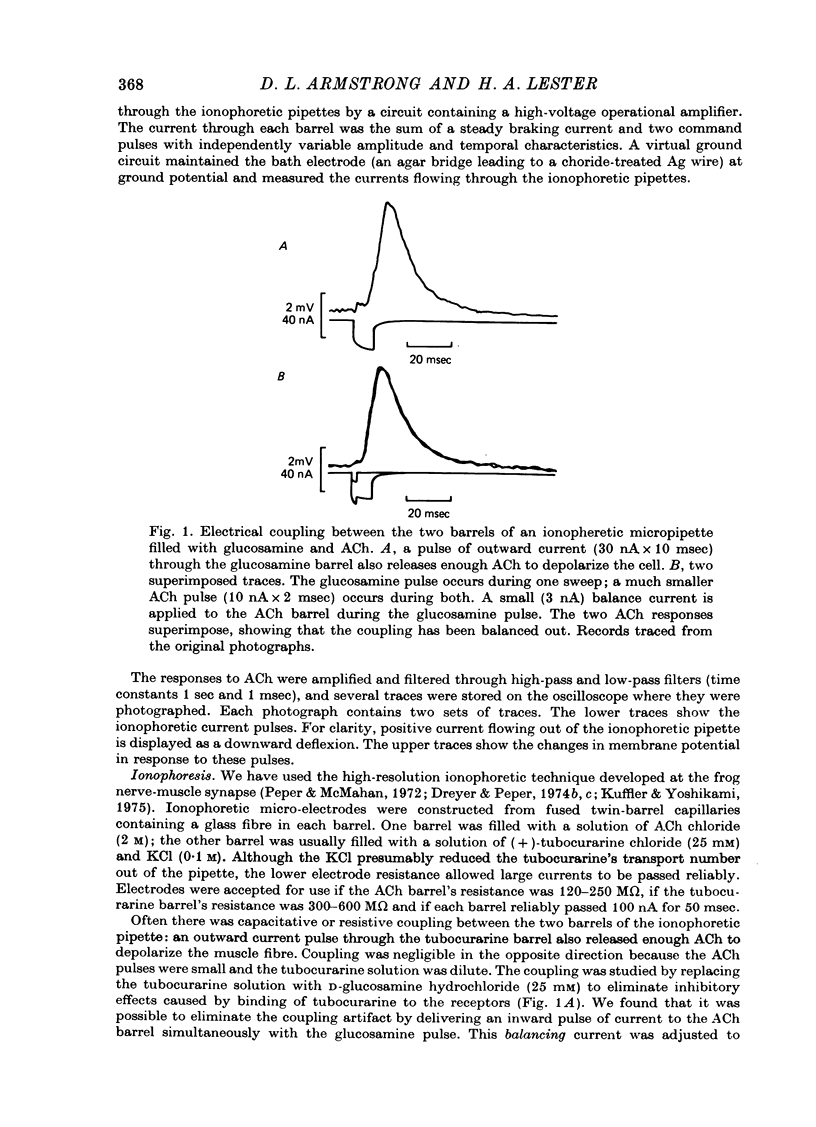

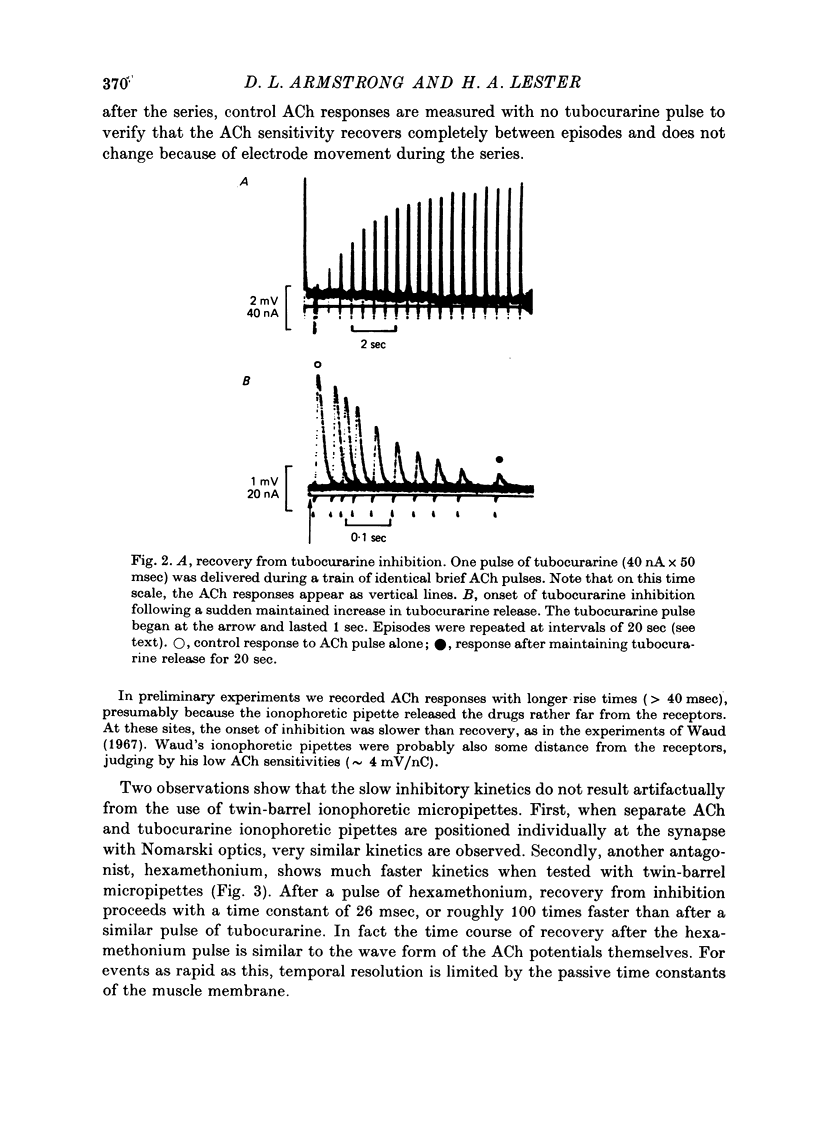
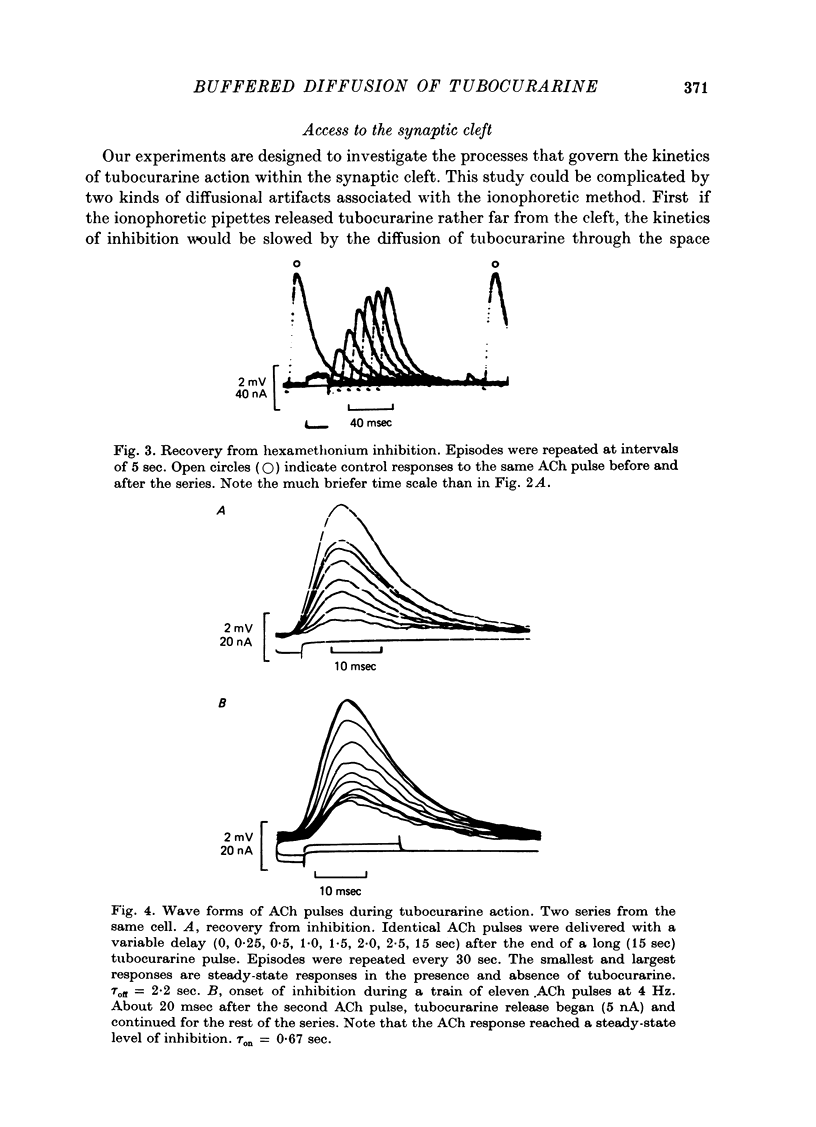
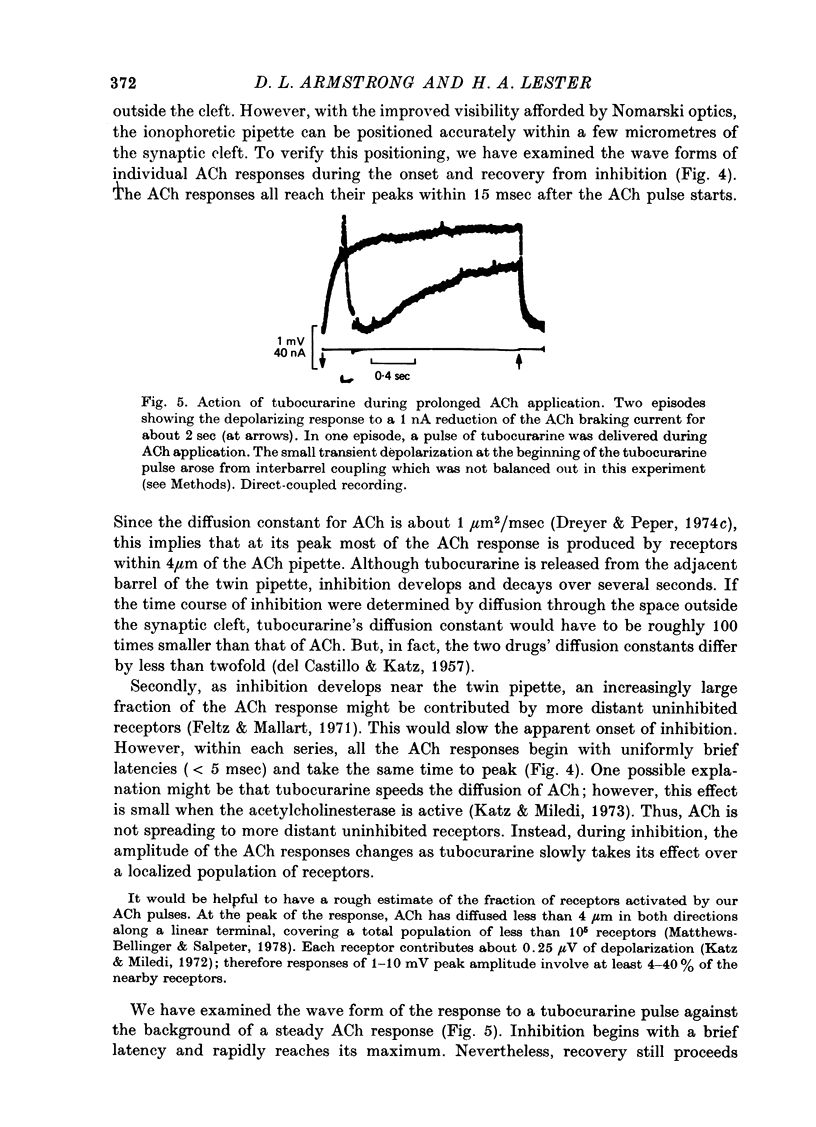


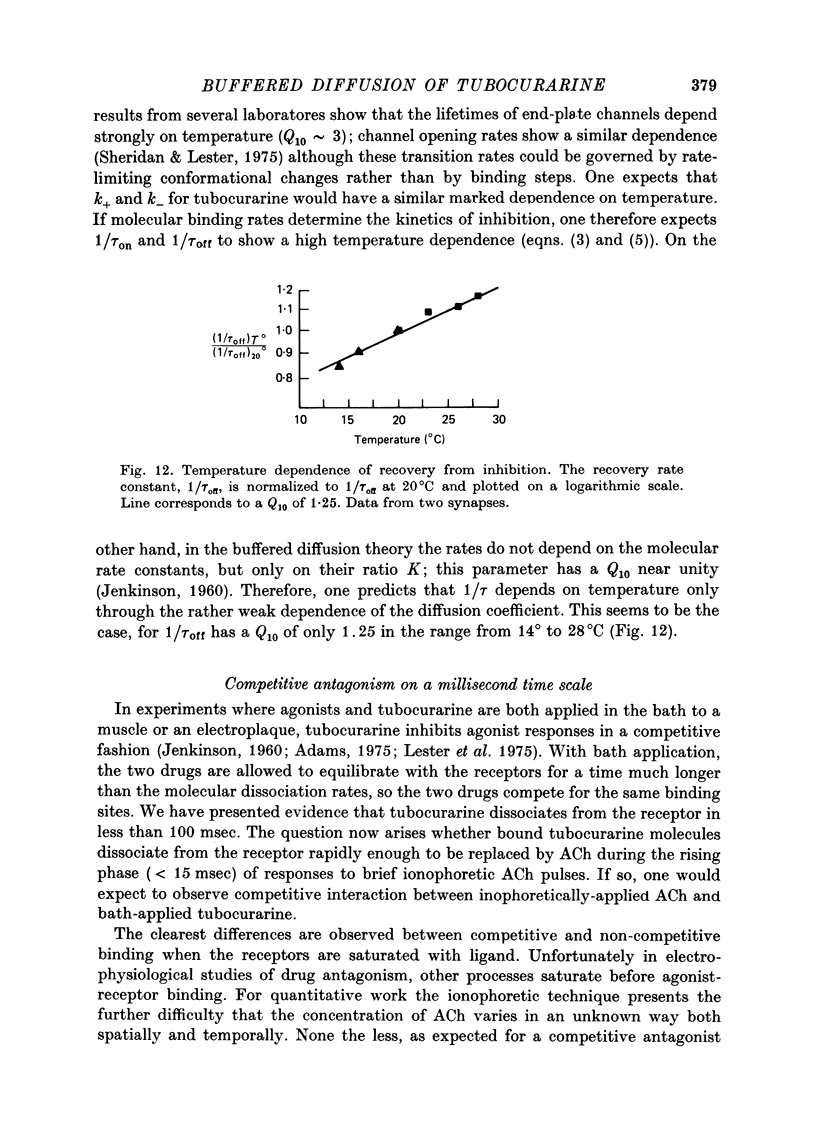






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
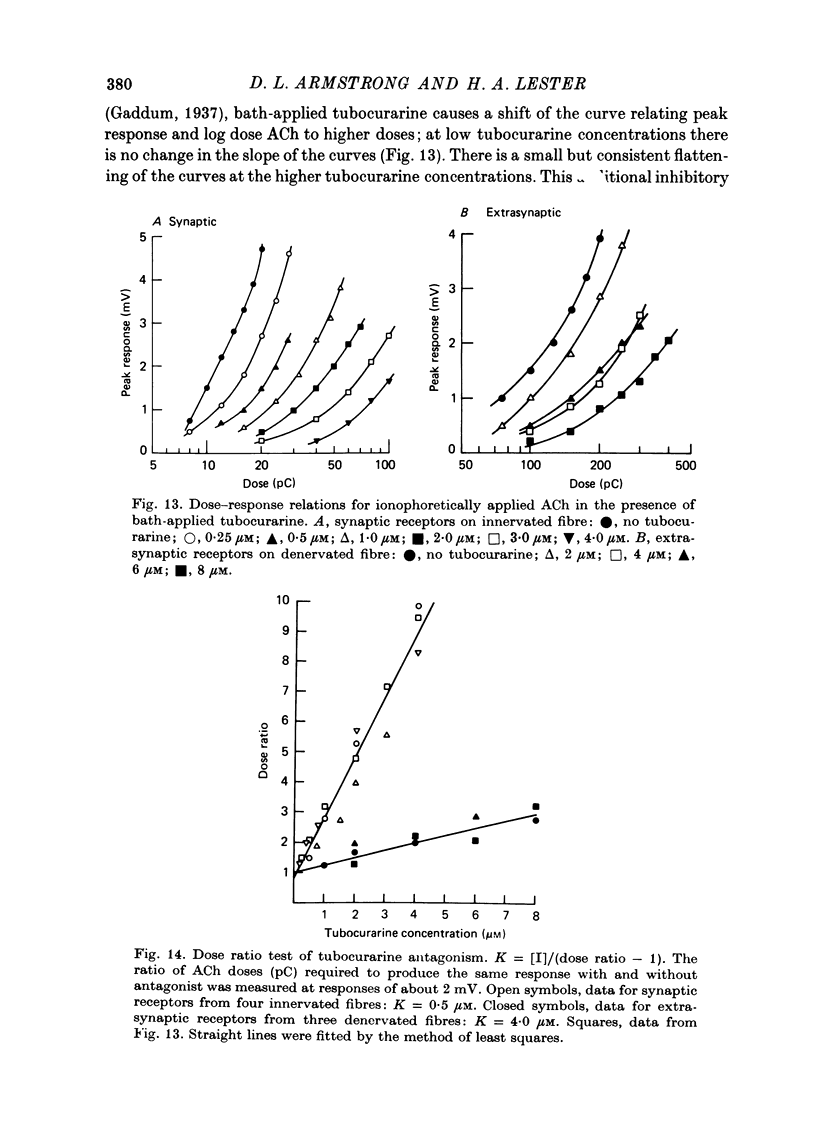
- Adams P. R. Drug interactions at the motor endplate. Pflugers Arch. 1975 Oct 28;360(2):155–164. doi: 10.1007/BF00580538. [DOI] [PubMed] [Google Scholar]
- Alper R., Lowy J., Schmidt J. Binding properties of acetylcholine receptors extracted from normal and from denervated rat diaphragm. FEBS Lett. 1974 Nov 1;48(1):130–132. doi: 10.1016/0014-5793(74)81079-3. [DOI] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W., Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol. 1973 May;230(3):673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beychok S. On the problem of isolation of the specific acetylcholine receptor. Biochem Pharmacol. 1965 Aug;14(8):1249–1255. doi: 10.1016/0006-2952(65)90302-3. [DOI] [PubMed] [Google Scholar]
- Blackman J. G., Gauldie R. W., Milne R. J. Interaction of competitive antagonists: the anti-curare action of hexamethonium and other antagonists at the skeletal neuromuscular junction. Br J Pharmacol. 1975 May;54(1):91–100. doi: 10.1111/j.1476-5381.1975.tb07414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. II. Comparison of junctional and extrajunctional receptors. Biochemistry. 1975 May 20;14(10):2100–2106. doi: 10.1021/bi00681a009. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The action of tubocurarine at the neuromuscular junction [proceedings]. J Physiol. 1978 Nov;284:171P–172P. [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Range H. P. Effects of inhibitors of the binding of iodinated alpha-bungarotoxin to acetylcholine receptors in rat muscle. Mol Pharmacol. 1976 Jul;12(4):519–535. [PubMed] [Google Scholar]
- DEL CASTILLO L., KATZ B. A study of curare action with an electrical micromethod. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):339–356. doi: 10.1098/rspb.1957.0015. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. A monolayer preparation of innervated skeletal muscle fibres of the m. cutaneus pectoris of the frog. Pflugers Arch. 1974 Apr 22;348(3):257–262. doi: 10.1007/BF00587416. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Density and dose-response curve of acetylcholine receptors in frog neuromuscular junction. Nature. 1975 Feb 20;253(5493):641–643. doi: 10.1038/253641a0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The acetylcholine sensitivity in the vicinity of the neuromuscular junction of the frog. Pflugers Arch. 1974 May 6;348(4):273–286. doi: 10.1007/BF00589217. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol. 1909 Dec 23;39(5):361–373. doi: 10.1113/jphysiol.1909.sp001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson E., Arnberg H., Eaker D. Isolation of the principal neurotoxins of two Naja naja subspecies. Eur J Biochem. 1971 Jul 15;21(1):1–16. doi: 10.1111/j.1432-1033.1971.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Bezanilla F., Taylor R. E., Rojas E. The rate of action of tetrodotoxin on sodium conductance in the squid giant axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):365–375. doi: 10.1098/rstb.1975.0016. [DOI] [PubMed] [Google Scholar]
- Kordás M., Brzin M., Majcen Z. A comparison of the effect of cholinesterase inhibitors on end-plate current and on cholinesterase activity in frog muscle. Neuropharmacology. 1975 Nov;14(11):791–800. doi: 10.1016/0028-3908(75)90106-9. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Podleski T. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor distribution on myotubes in culture correlated to acetylcholine sensitivity. J Physiol. 1977 Jul;269(1):155–176. doi: 10.1113/jphysiol.1977.sp011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J Physiol. 1905 Dec 30;33(4-5):374–413. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. Blockade of acetylcholine receptors by cobra toxin: electrophysiological studies. Mol Pharmacol. 1972 Nov;8(6):623–631. [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T., Schmidt J., Raftery M. A. Binding of acetylcholine and related compounds to purified acetylcholine receptor from Torpedo Californica electroplax. Biochem Biophys Res Commun. 1973 Aug 6;53(3):761–772. doi: 10.1016/0006-291x(73)90158-7. [DOI] [PubMed] [Google Scholar]
- Mooser G., Sigman D. S. Ligand binding properties of acetylcholinesterase determined with fluorescent probes. Biochemistry. 1974 May 21;13(11):2299–2307. doi: 10.1021/bi00708a010. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Waud D. R. The margin of safety of neuromuscular transmission. J Physiol. 1967 Jul;191(1):59–90. doi: 10.1113/jphysiol.1967.sp008237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves R. D. The time course of cellular responses to iontophoretically applied drugs. J Theor Biol. 1977 Mar 21;65(2):327–344. doi: 10.1016/0022-5193(77)90328-9. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P., Lappi S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry. 1975 May 6;14(9):1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- Thron C. D., Waud D. R. The rate of action of atropine. J Pharmacol Exp Ther. 1968 Mar;160(1):91–105. [PubMed] [Google Scholar]
- Waud D. R. The rate of action of competitive neuromuscular blocking agents. J Pharmacol Exp Ther. 1967 Oct;158(1):99–114. [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. II. Effect of cholinergic agonists and antagonists on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):15–34. [PubMed] [Google Scholar]
- Weber M., David-Pfeuty T., Changeux J. P. Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3443–3447. doi: 10.1073/pnas.72.9.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Lappi S., Chignell C. F., Taylor P. Kinetics of agonist-mediated transitions in state of the cholinergic receptor. J Biol Chem. 1977 Nov 10;252(21):7648–7656. [PubMed] [Google Scholar]


