Abstract
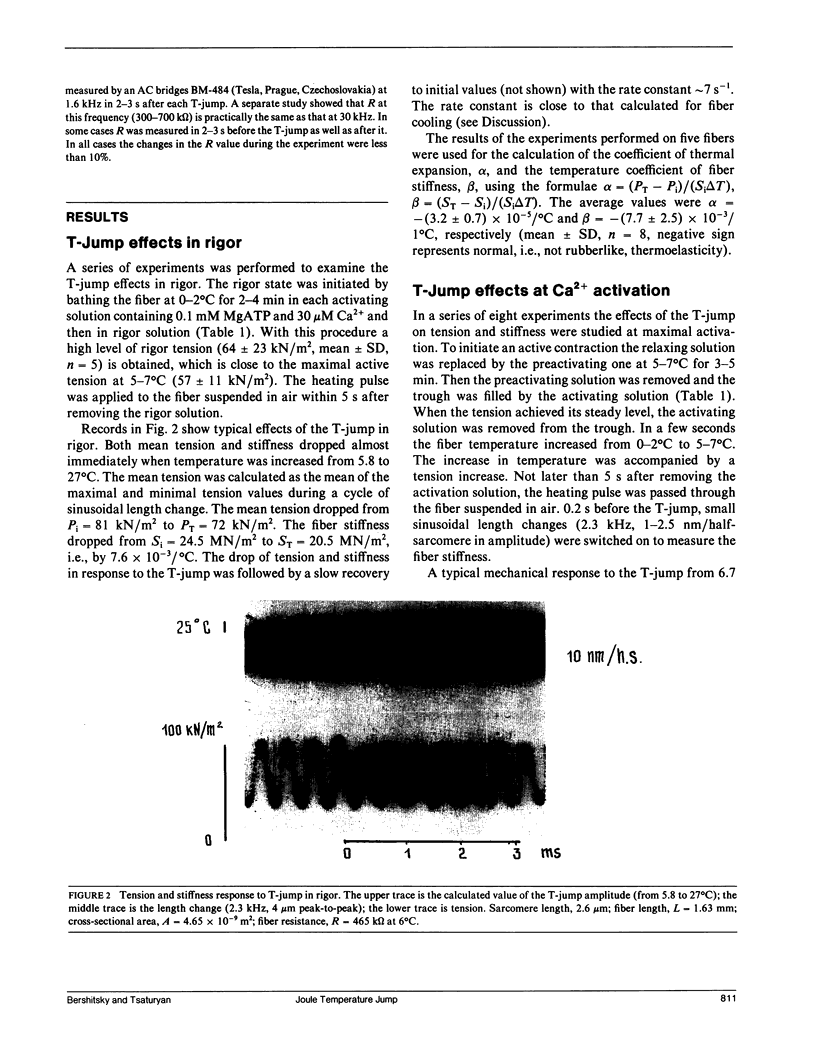
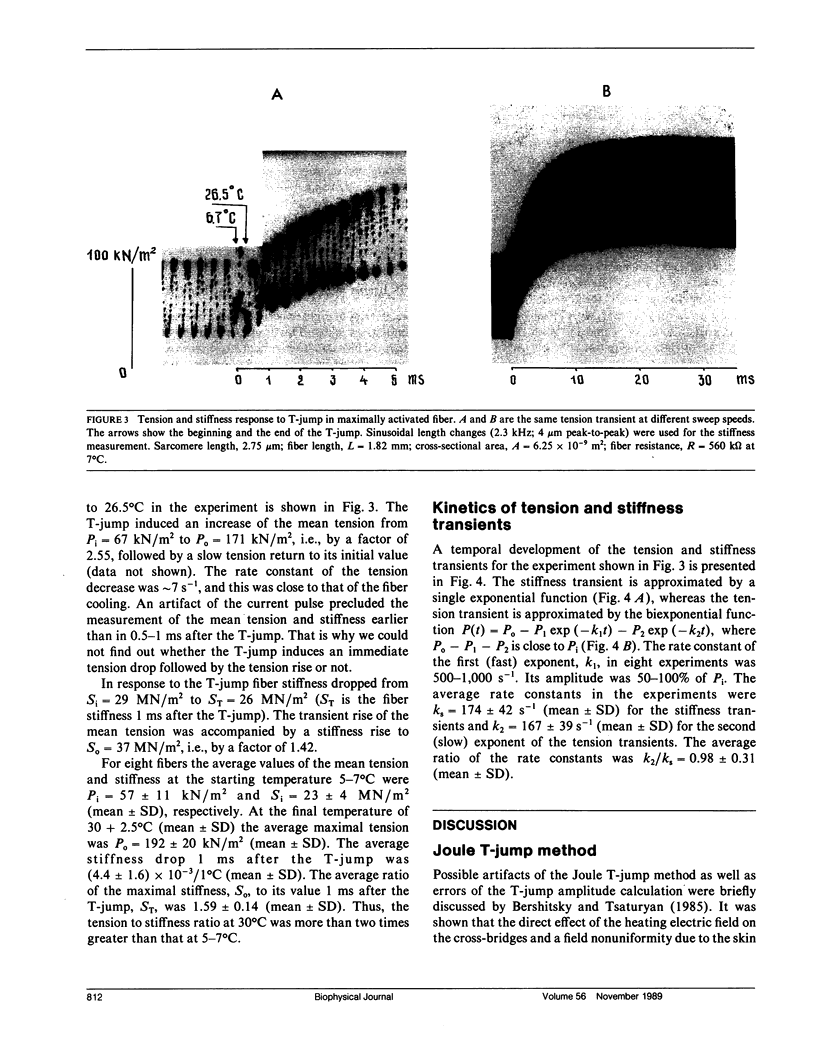
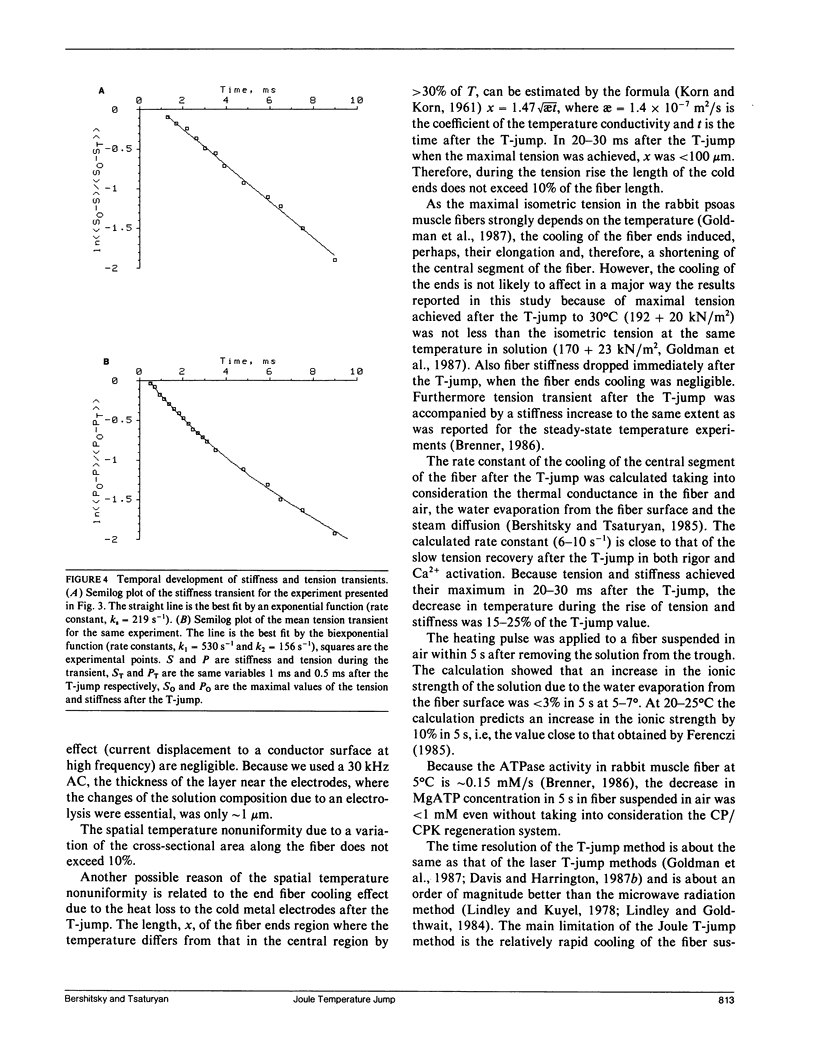
The effects of a temperature jump (T-jump) from 5-7 degrees C to 26-33 degrees C were studied on tension and stiffness of glycerol-extracted fibers from rabbit psoas muscle in rigor and during maximal Ca2+ activation. The T-jump was initiated by passing an alternating current pulse (30 kHz, up to 2.5 kV, duration 0.2 ms) through a fiber suspended in air. In rigor the T-jump induces a drop of both tension and stiffness. During maximal activation, the immediate stiffness dropped by (4.4 +/- 1.6) x 10(-3)/1 degree C (mean + SD) in response to the T-jump, and this was followed by a monoexponential stiffness rise by a factor of 1.59 +/- 0.14 with a rate constant ks = 174 +/- 42 s-1 (mean +/- SD, n = 8). The data show that the fiber stiffness, determined by the cross-bridge elasticity, in both rigor and maximal activation is not rubber-like. In the activated fibers the T-jump induced a biexponential tension rise by a factor of 3.45 +/- 0.76 (mean +/- SD, n = 8) with the rate constants 500-1,000 s-1 for the first exponent and 167 +/- 39 s-1 (mean +/- SD, n = 8) for the second exponent. The data are in accordance with the assumption that the first phase of the tension transient after the T-jump is due to a force-generating step in the attached cross-bridges, whereas the second one is related to detachment and reattachment of cross-bridges.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res Cardiol. 1986;81 (Suppl 1):1–15. doi: 10.1007/978-3-662-11374-5_1. [DOI] [PubMed] [Google Scholar]
- Chiu Y. L., Karwash S., Ford L. E. A piezoelectric force transducer for single muscle cells. Am J Physiol. 1978 Sep;235(3):C143–C146. doi: 10.1152/ajpcell.1978.235.3.C143. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Harrington W. F. Force generation by muscle fibers in rigor: a laser temperature-jump study. Proc Natl Acad Sci U S A. 1987 Feb;84(4):975–979. doi: 10.1073/pnas.84.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S., Harrington W. F. Laser temperature-jump apparatus for the study of force changes in fibers. Anal Biochem. 1987 Mar;161(2):543–549. doi: 10.1016/0003-2697(87)90487-8. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Proceedings: Mechanism of early tension recovery after a quick release in tetanized muscle fibres. J Physiol. 1974 Jul;240(2):42P–43P. [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. H., Ford L. E. The thermoelastic effect in rigor muscle of the frog. J Muscle Res Cell Motil. 1986 Feb;7(1):35–46. doi: 10.1007/BF01756200. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., McCray J. A., Ranatunga K. W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol. 1987 Nov;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F. A mechanochemical mechanism for muscle contraction. Proc Natl Acad Sci U S A. 1971 Mar;68(3):685–689. doi: 10.1073/pnas.68.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F. On the origin of the contractile force in skeletal muscle. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5066–5070. doi: 10.1073/pnas.76.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kimura M., Tawada K. Is the SII portion of the cross-bridge in glycerinated rabbit psoas fibers compliant in the rigor state? Biophys J. 1984 Mar;45(3):603–610. doi: 10.1016/S0006-3495(84)84198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometani K., Yamada K. Thermoelastic effect in chemically skinned frog skeletal muscle in rigor. Jpn J Physiol. 1984;34(3):389–396. doi: 10.2170/jjphysiol.34.389. [DOI] [PubMed] [Google Scholar]
- Lindley B. D., Kuyel B. Contractile deactivation by rapid, microwave-induced temperature jumps. Biophys J. 1978 Oct;24(1):254–255. doi: 10.1016/S0006-3495(78)85367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Morozov V. N., Morozova T. Y. Viscoelastic properties of protein crystals: triclinic crystals of hen egg white lysozyme in different conditions. Biopolymers. 1981 Mar;20(3):451–467. doi: 10.1002/bip.1981.360200304. [DOI] [PubMed] [Google Scholar]
- Tawada K., Kimura M. Stiffness of carbodiimide-crosslinked glycerinated muscle fibres in rigor and relaxing solutions at high salt concentrations. J Muscle Res Cell Motil. 1986 Aug;7(4):339–350. doi: 10.1007/BF01753655. [DOI] [PubMed] [Google Scholar]
- Tawada K., Kimura M. Stiffness of glycerinated rabbit psoas fibers in the rigor state. Filament-overlap relation. Biophys J. 1984 Mar;45(3):593–602. doi: 10.1016/S0006-3495(84)84197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]




