Abstract
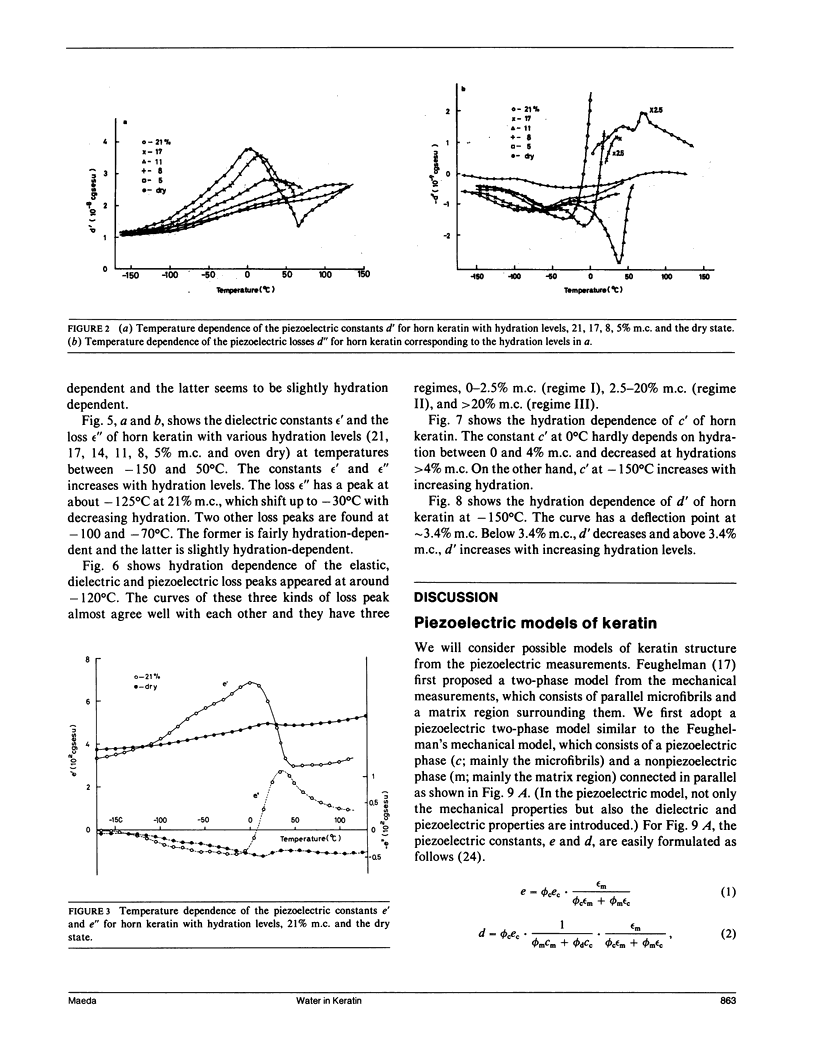
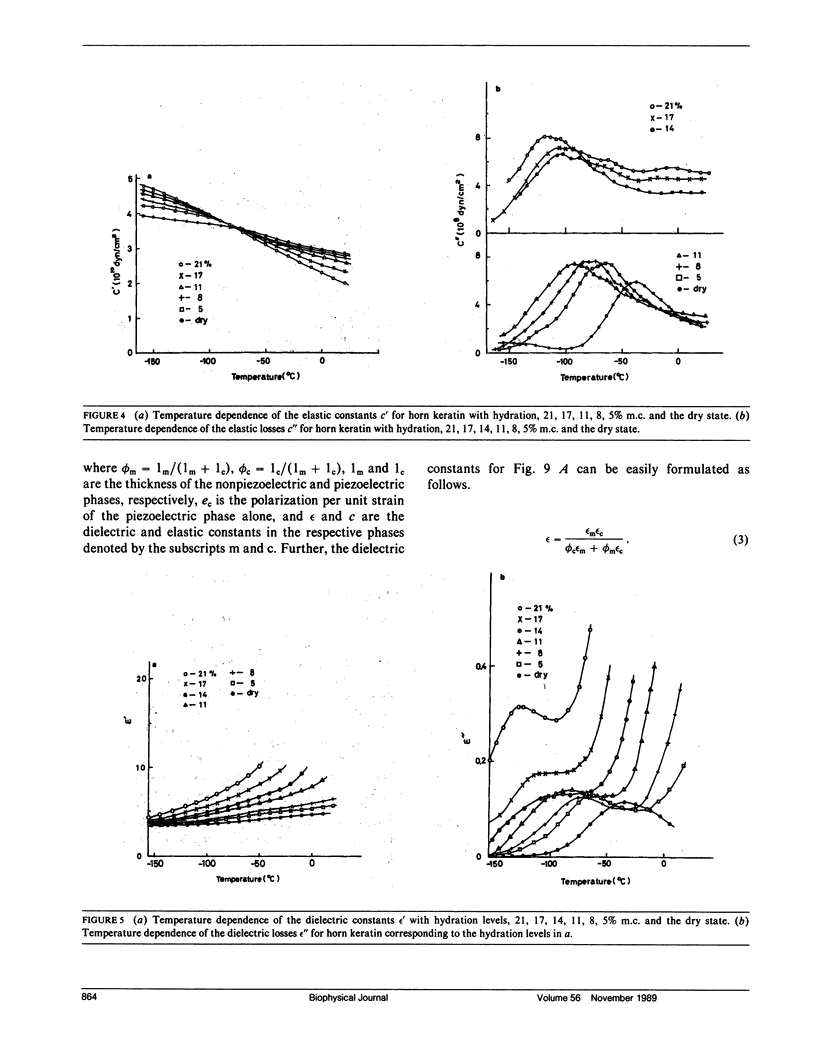
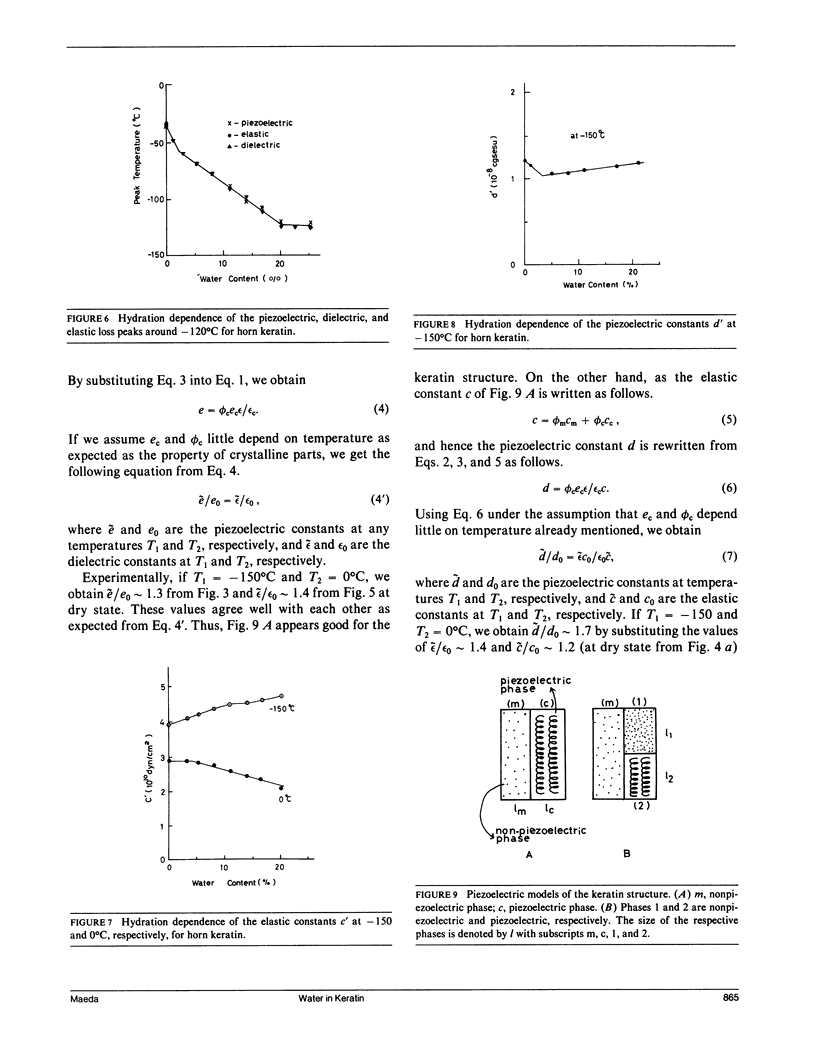
To investigate actions of water in keratin, the piezoelectric, dielectric, and elastic constants are measured at 10 Hz, at temperatures between -160 and 150 degrees C, and at various hydration levels. From changes in the piezoelectric, dielectric, and dynamic mechanical parameters with moisture content (m.c.), we have identified three regimes (I, II, and III) in the hydration of water for keratin. At high hydration (21% m.c.) around 0 degree C, the piezoelectric constants for keratin steeply decrease with increasing temperature. This may be attributed to interfacial polarization which is strongly related to self-associated water molecules (particularly regime III water) just around crystalline helical regions which can exhibit the stress-induced, i.e., piezoelectric, polarization and may be attributed to electrode polarization induced by the increase of mobile ions in the amorphous matrix region, some of which would be released from their trapped states just around the piezoelectric phase by the regime III water. With increasing hydration, the elastic constants for keratin are found to increase below -70 degrees C and decrease above -70 degrees C. This suggests a viscoelastic transition of the keratin structure due to bound water (regime II water). The piezoelectric, dielectric, and elastic loss peaks are found at around -120 degrees C for hydrated keratin, believed to be due to tightly bound water (regime I water), which acts only to stiffen the keratin structure. The adsorption regions of water in keratin are discussed by a piezoelectric two-phase model, which consists of piezoelectric and nonpiezoelectric phases. It is proposed that water molecule would at least adsorb in the nonpiezoelectric phase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fraser R. D., MacRae T. P., Suzuki E. Structure of the alpha-keratin microfibril. J Mol Biol. 1976 Dec;108(2):435–452. doi: 10.1016/s0022-2836(76)80129-5. [DOI] [PubMed] [Google Scholar]
- Lynch L. J., Marsden K. H. NMR of absorbed systems. II. A NMR study of keratin hydration. J Chem Phys. 1969 Dec 15;51(12):5681–5691. doi: 10.1063/1.1671998. [DOI] [PubMed] [Google Scholar]
- Maeda H., Fukada E. Effect of water on piezoelectric, dielectric, and elastic properties of bone. Biopolymers. 1982 Oct;21(10):2055–2068. doi: 10.1002/bip.360211010. [DOI] [PubMed] [Google Scholar]
- Sasaki N. Dielectric properties of slightly hydrated collagen: time-water content superposition analysis. Biopolymers. 1984 Sep;23(9):1724–1734. doi: 10.1002/bip.360230908. [DOI] [PubMed] [Google Scholar]


