Abstract
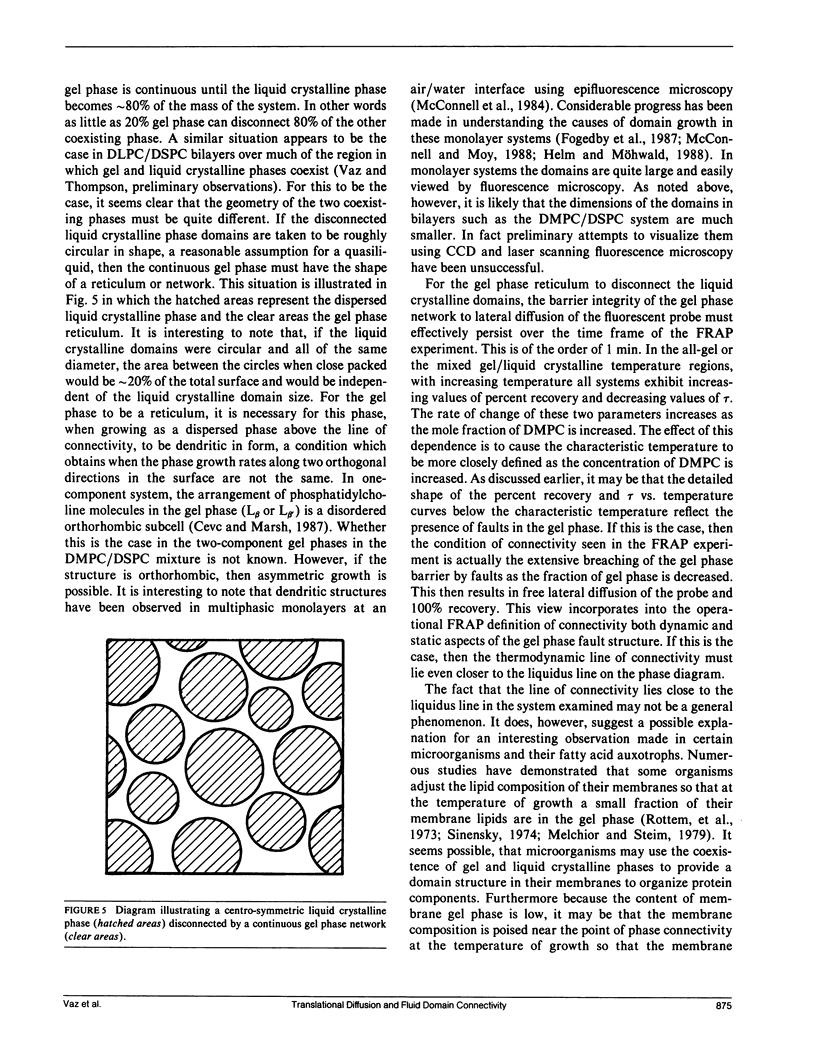
The two-dimensional connectivity is examined for mixed bilayers of dimyristoyl phosphatidylcholine (DMPC) and distearoyl phosphatidylcholine (DSPC) as a function of composition and temperature at constant pressure using the fluorescence recovery after photobleaching (FRAP) method. These phospholipid mixtures exhibit peritectic behavior with a large region in which both gel and liquid crystalline phases coexist. Dilauroyl phosphatidylethanolamine covalently linked through the amino function in its head group to the fluorescent nitrobenzodiazolyl group (NBD-DLPE) was used as the fluorescent probe in this study, because it was found to partition almost exclusively in the liquid crystalline phase. The results of these studies show the line of connectivity to be close to the liquidus line on the phase diagram over a rather broad range of concentrations. In this range, a gel phase comprising approximately 20% of the system disconnects a liquid crystalline phase comprising 80% of the system. The implications of this result are discussed for domain shape and the organization of biological membrane components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A., Wittebort R. J., Das Gupta S. K., Griffin R. G. Phase equilibria, molecular conformation, and dynamics in phosphatidylcholine/phosphatidylethanolamine bilayers. Biochemistry. 1982 Nov 23;21(24):6243–6253. doi: 10.1021/bi00267a032. [DOI] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979 May 15;18(10):2112–2117. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Gaub H., Sackmann E., Büschl R., Ringsdorf H. Lateral diffusion and phase separation in two-dimensional solutions of polymerized butadiene lipid in dimyristoylphosphatidylcholine bilayers. A photobleaching and freeze fracture study. Biophys J. 1984 Apr;45(4):725–731. doi: 10.1016/S0006-3495(84)84215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W. Geometry of phase-separated domains in phospholipid bilayers by diffraction-contrast electron microscopy. Biophys J. 1981 Jun;34(3):383–395. doi: 10.1016/S0006-3495(81)84857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Wolf D. E. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry. 1980 Dec 23;19(26):6199–6203. doi: 10.1021/bi00567a039. [DOI] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Cirillo V. P., de Kruyff B., Shinitzky M., Razin S. Cholesterol in mycoplasma membranes. Correlation of enzymic and transport activities with physical state of lipids in membranes of Mycoplasma mycoides var. capri adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):509–519. doi: 10.1016/0005-2736(73)90159-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. B., Chan W. K., Webb W. W. Fast diffusion along defects and corrugations in phospholipid P beta, liquid crystals. Biophys J. 1983 Aug;43(2):157–165. doi: 10.1016/S0006-3495(83)84336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Snyder B., Freire E. Compositional domain structure in phosphatidylcholine--cholesterol and sphingomyelin--cholesterol bilayers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4055–4059. doi: 10.1073/pnas.77.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumpasis D. M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J. 1983 Jan;41(1):95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugár I. P., Monticelli G. Interrelationships between the phase diagrams of the two-component phospholipid bilayers. Biophys J. 1985 Aug;48(2):283–288. doi: 10.1016/S0006-3495(85)83781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]


