Abstract
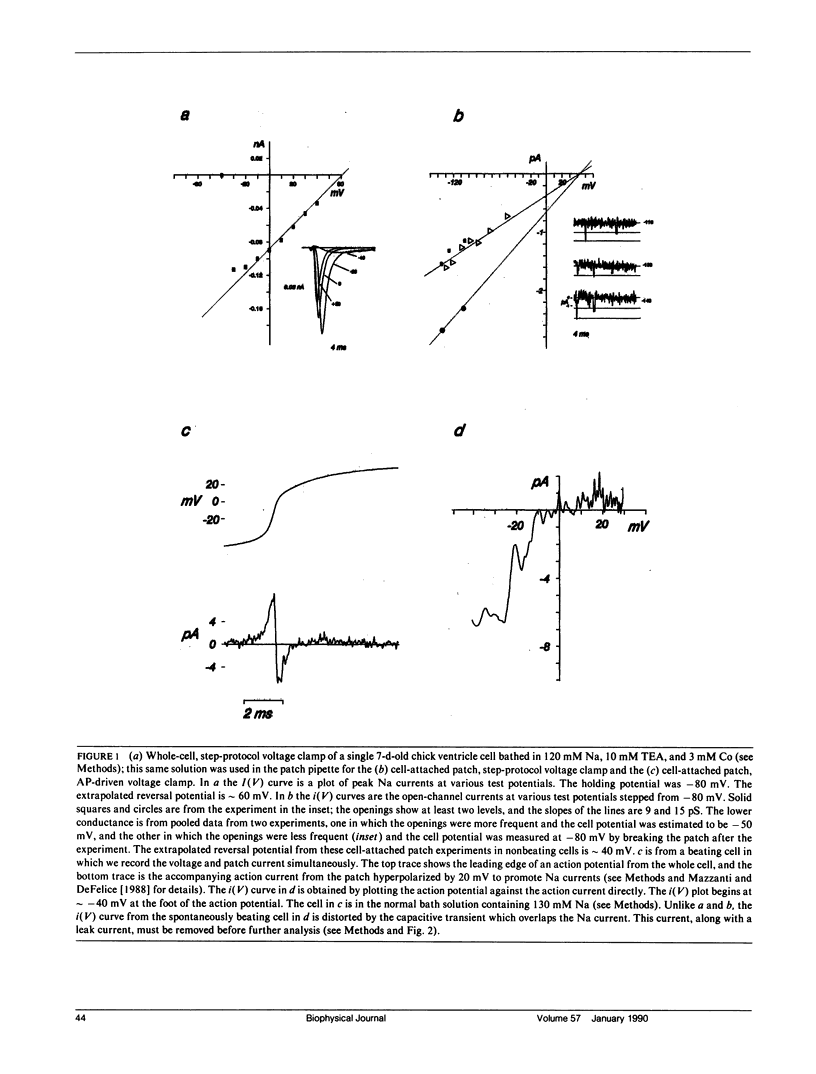
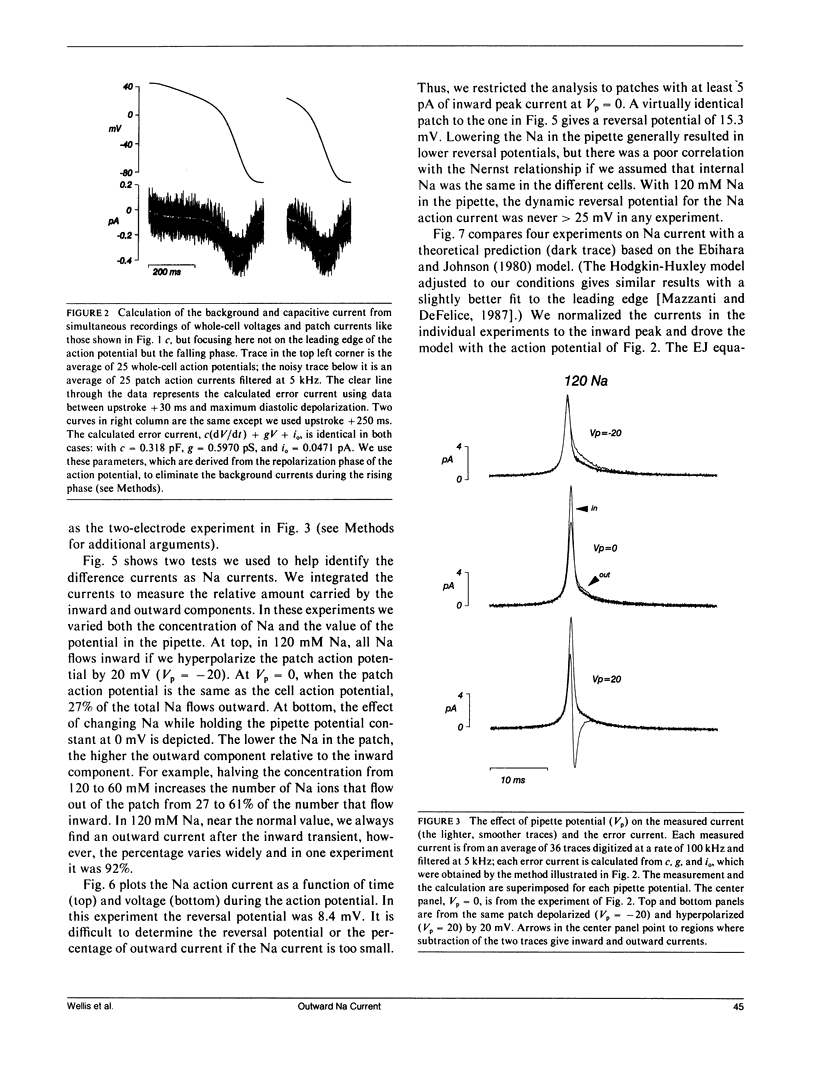
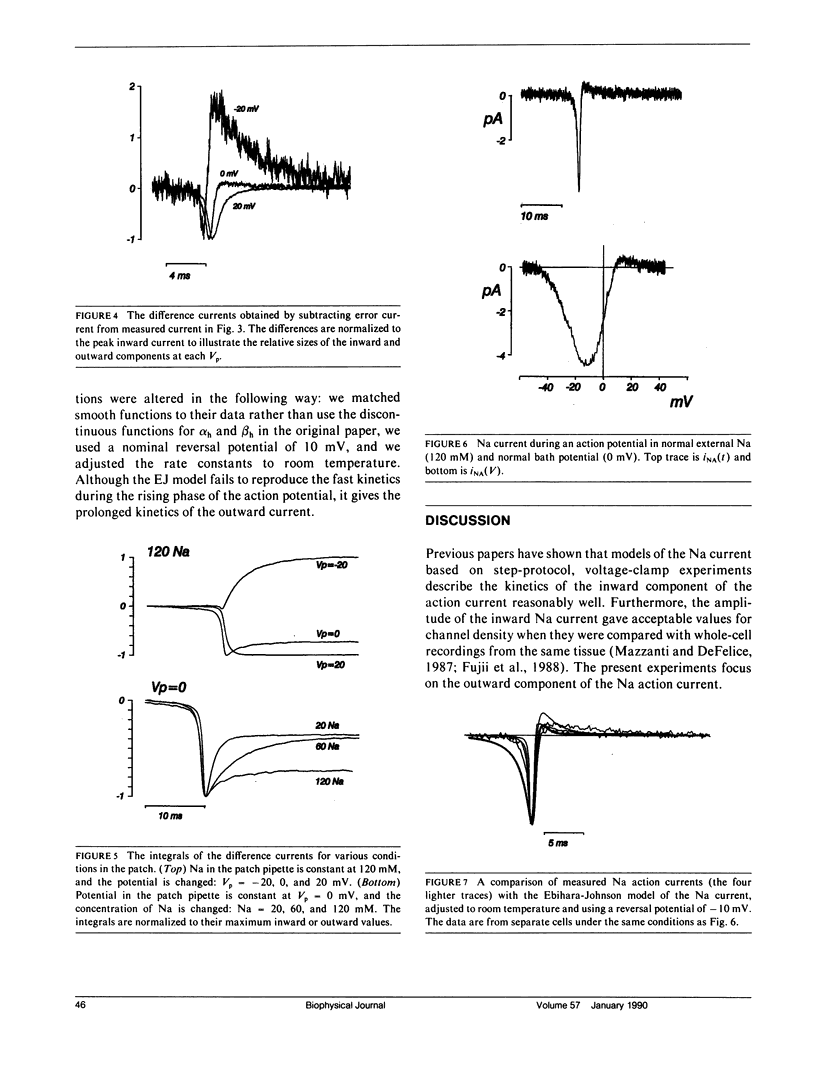
This article is a study of the fast Na current during action potentials. We have investigated the outward Na current (Mazzanti, M., and L.J. DeFelice. 1987. Biophys. J. 52:95-100) in more detail, and we have asked whether it goes through the same channels associated with the rapid depolarization phase of action potentials. We address the question by patch clamping single, spontaneously beating, embryonic chick ventricle cells, using two electrodes to record the action potential and the patch current simultaneously. The chief limitation is the capacitive current, and in this article we describe a new method to subtract it. Varying the potential and the Na concentration in the patch pipette, and fitting the corrected currents to a standard model (Ebihara, L., and E.A. Johnson. 1980. Biophys. J. 32:779-790), provides evidence that the outward current is carried by the same channels that conduct the inward current. We compare the currents in beating cells to currents in nonbeating cells using whole-cell and cell-attached patch clamp recordings. The latter tend to show more positive Na reversal potentials, with the implication that internal Na is higher in beating cells. We propose that the plateau of the action potential, which is partly due to an inward Ca current, exceeds Na action current reversal potentials, and that this driving force gives rise to an outward movement of Na ions. The existence of such a current would imply that the fast repolarization phase after the upstroke of cardiac action potentials is partly due to the Na action current.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeHann R. L. Regulation of spontaneous activity and growth of embryonic chick heart cells in tissue culture. Dev Biol. 1967 Sep;16(3):216–249. doi: 10.1016/0012-1606(67)90025-5. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follmer C. H., ten Eick R. E., Yeh J. Z. Sodium current kinetics in cat atrial myocytes. J Physiol. 1987 Mar;384:169–197. doi: 10.1113/jphysiol.1987.sp016449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Hanck D. A., Makielski J. C., Scanley B. E., Sheets M. F. Sodium channels in cardiac Purkinje cells. Experientia. 1987 Dec 1;43(11-12):1162–1168. doi: 10.1007/BF01945516. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Sheu S. S. Intracellular potassium and sodium activities of chick ventricular muscle during embryonic development. J Physiol. 1980 Sep;306:579–586. doi: 10.1113/jphysiol.1980.sp013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Ayer R. K., Jr, DeHaan R. L. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988 Mar;101(3):209–223. doi: 10.1007/BF01872836. [DOI] [PubMed] [Google Scholar]
- Kell M. J., DeFelice L. J. Surface charge near the cardiac inward-rectifier channel measured from single-channel conductance. J Membr Biol. 1988 Apr;102(1):1–10. doi: 10.1007/BF01875348. [DOI] [PubMed] [Google Scholar]
- Makielski J. C., Sheets M. F., Hanck D. A., January C. T., Fozzard H. A. Sodium current in voltage clamped internally perfused canine cardiac Purkinje cells. Biophys J. 1987 Jul;52(1):1–11. doi: 10.1016/S0006-3495(87)83182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. K channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1988 Dec;54(6):1139–1148. doi: 10.1016/S0006-3495(88)83048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Na channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1987 Jul;52(1):95–100. doi: 10.1016/S0006-3495(87)83192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., DeHaan R. L. Ion levels and membrane potential in chick heart tissue and cultured cells. J Gen Physiol. 1973 Jan;61(1):89–109. doi: 10.1085/jgp.61.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada H., Kojima M., Sperelakis N. Fast inward current properties of voltage-clamped ventricular cells of embryonic chick heart. Am J Physiol. 1988 Sep;255(3 Pt 2):H540–H553. doi: 10.1152/ajpheart.1988.255.3.H540. [DOI] [PubMed] [Google Scholar]
- Scanley B. E., Fozzard H. A. Low conductance sodium channels in canine cardiac Purkinje cells. Biophys J. 1987 Sep;52(3):489–495. doi: 10.1016/S0006-3495(87)83237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. F., Scanley B. E., Hanck D. A., Makielski J. C., Fozzard H. A. Open sodium channel properties of single canine cardiac Purkinje cells. Biophys J. 1987 Jul;52(1):13–22. doi: 10.1016/S0006-3495(87)83183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


