Abstract
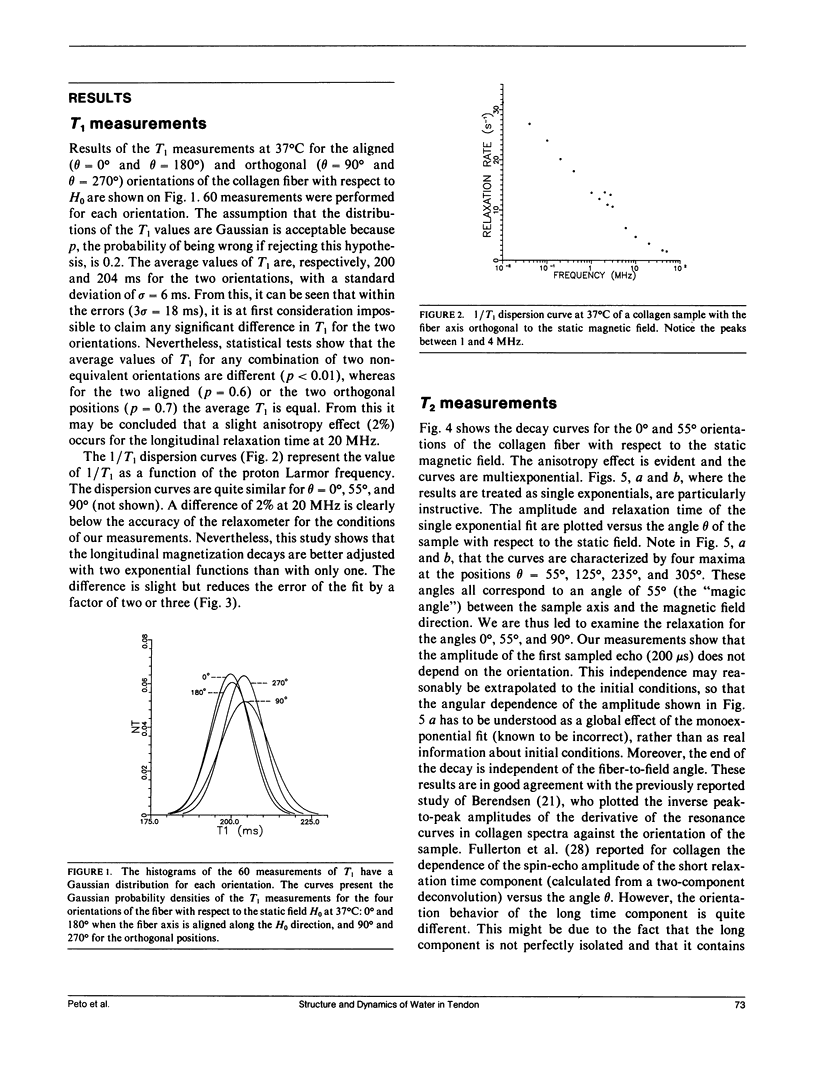
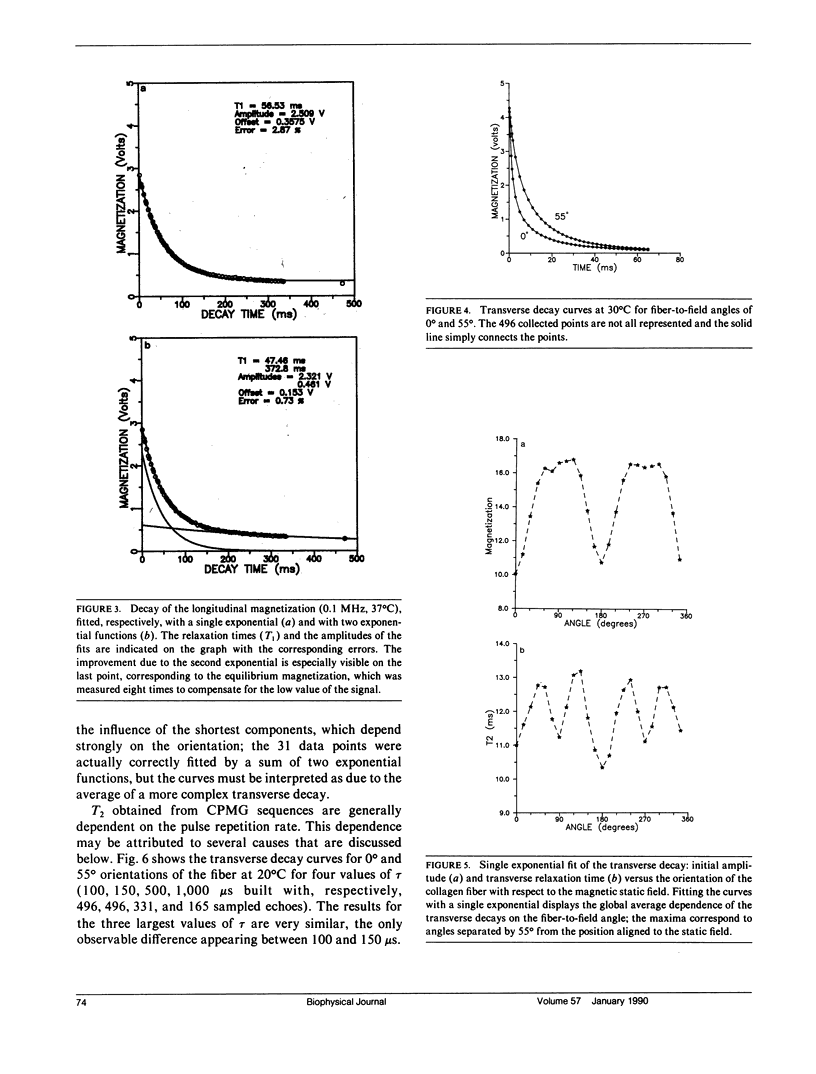
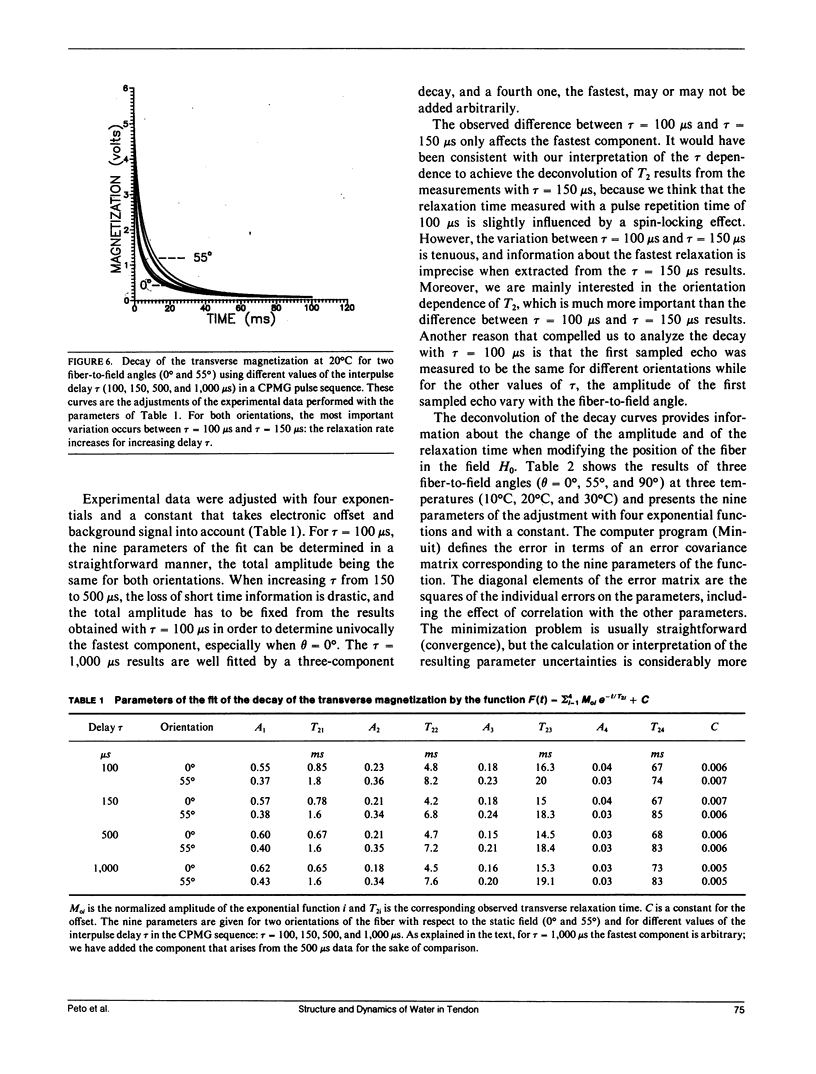
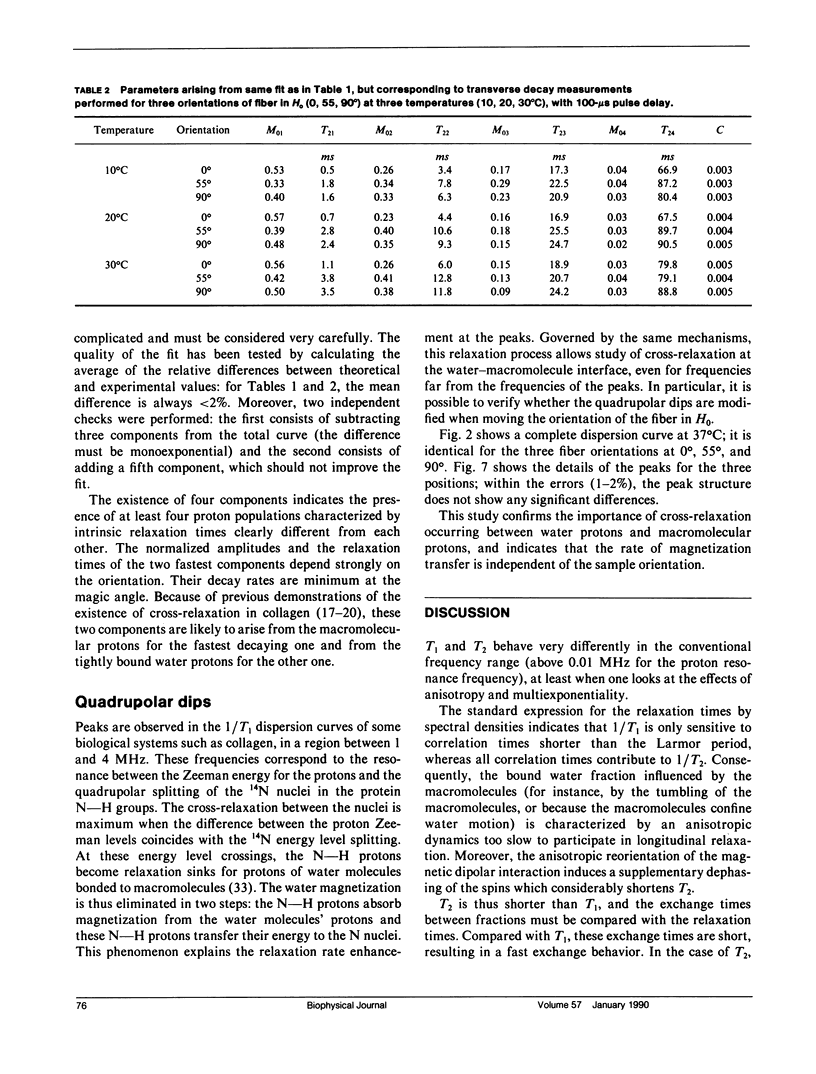
Nuclear magnetic relaxation times were measured in collagen tissue when varying the orientation of the fiber with respect to the static field. T1 was found to be only slightly dependent on theta, the fiber-to-field angle, but T2 was very sensitive to the orientation, with a maximum value at the magic angle. The transverse decay curves were multiexponential. Their deconvolution displayed four components; the ones that decayed most slowly were almost independent of theta, but the two fastest ones showed a strong angular dependence that was interpreted with a cross-relaxation model. Quadrupolar dips were visible in the 1/T1 dispersion curves. These dips were independent of theta, so that the magnetization transfer could also be assumed to be independent of the fiber orientation. Finally, each component was assigned to a fraction of protons localized in the macromolecular structure and characterized by particular dynamics. The model of Woessner was applied to the water molecules tightly bound into the macromolecules, which resulted in a dynamical description of this water fraction. This description is compatible with the two-sites model of Ramachandran based on x-ray diffraction and with the extensive studies of Berendsen. However, the important indications obtained from the deconvolution lead to a less static representation of the tissue.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLERHAND A., GUTOWSKY H. S. SPIN-ECHO STUDIES OF CHEMICAL EXCHANGE. II. CLOSED FORMULAS FOR TWO SITES. J Chem Phys. 1965 Mar 1;42:1587–1599. doi: 10.1063/1.1696165. [DOI] [PubMed] [Google Scholar]
- Cleveland G. G., Chang D. C., Hazlewood C. F., Rorschach H. E. Nuclear magnetic resonance measurement of skeletal muscle: anisotrophy of the diffusion coefficient of the intracellular water. Biophys J. 1976 Sep;16(9):1043–1053. doi: 10.1016/S0006-3495(76)85754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Wien R. The state of water in muscle tissue as determined by proton nuclear magnetic resonance. Biophys J. 1971 Dec;11(12):1002–1017. doi: 10.1016/S0006-3495(71)86274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehl R. E. Collagen: mobile water content of frozen fibers. Science. 1970 Nov 13;170(3959):738–739. doi: 10.1126/science.170.3959.738. [DOI] [PubMed] [Google Scholar]
- Dehl R. E., Hoeve C. A. Broad-line NMR study of H2O and D2O in collagen fibers. J Chem Phys. 1969 Apr 15;50(8):3245–3251. doi: 10.1063/1.1671547. [DOI] [PubMed] [Google Scholar]
- Edzes H. T., Samulski E. T. Cross relaxation and spin diffusion in the proton NMR or hydrated collagen. Nature. 1977 Feb 10;265(5594):521–523. doi: 10.1038/265521a0. [DOI] [PubMed] [Google Scholar]
- Fullerton G. D., Cameron I. L., Ord V. A. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiology. 1985 May;155(2):433–435. doi: 10.1148/radiology.155.2.3983395. [DOI] [PubMed] [Google Scholar]
- Fullerton G. D., Ord V. A., Cameron I. L. An evaluation of the hydration of lysozyme by an NMR titration method. Biochim Biophys Acta. 1986 Feb 14;869(3):230–246. doi: 10.1016/0167-4838(86)90063-4. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Correlation of relaxation time with water content in muscle and brain tissues. Biochim Biophys Acta. 1977 Mar 29;497(1):317–322. doi: 10.1016/0304-4165(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Nuclear magnetic resonance study of water interactions with proteins, biomolecules, membranes, and tissues. Methods Enzymol. 1986;127:151–161. doi: 10.1016/0076-6879(86)27013-5. [DOI] [PubMed] [Google Scholar]
- Fung B. M., Puon P. S. Nuclear magnetic resonance transverse relaxation in muscle water. Biophys J. 1981 Jan;33(1):27–37. doi: 10.1016/S0006-3495(81)84870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood C. F., Chang D. C., Nichols B. L., Woessner D. E. Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys J. 1974 Aug;14(8):583–606. doi: 10.1016/S0006-3495(74)85937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood C. F., Nichols B. L., Chamberlain N. F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969 May 24;222(5195):747–750. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
- Kasturi S. R., Chang D. C., Hazlewood C. F. Study of anisotropy in nuclear magnetic resonance relaxation times of water protons in skeletal muscle. Biophys J. 1980 Jun;30(3):369–381. doi: 10.1016/S0006-3495(80)85102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Bryant R. G., Hallenga K., Jacob G. S. Magnetic cross-relaxation among protons in protein solutions. Biochemistry. 1978 Oct 3;17(20):4348–4358. doi: 10.1021/bi00613a037. [DOI] [PubMed] [Google Scholar]
- Koenig S. H. Theory of relaxation of mobile water protons induced by protein NH moieties, with application to rat heart muscle and calf lens homogenates. Biophys J. 1988 Jan;53(1):91–96. doi: 10.1016/S0006-3495(88)83069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran G. N., Chandrasekharan R. Interchain hydrogen bonds via bound water molecules in the collagen triple helix. Biopolymers. 1968;6(11):1649–1658. doi: 10.1002/bip.1968.360061109. [DOI] [PubMed] [Google Scholar]
- Renou J. P., Alizon J., Dohri M., Robert H. Study of the water--collagen system by NMR cross relaxation experiments. J Biochem Biophys Methods. 1983 Feb;7(2):91–99. doi: 10.1016/0165-022x(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Winter F., Kimmich R. 14N1H and 2H1H cross-relaxation in hydrated proteins. Biophys J. 1985 Aug;48(2):331–335. doi: 10.1016/S0006-3495(85)83787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


