Abstract
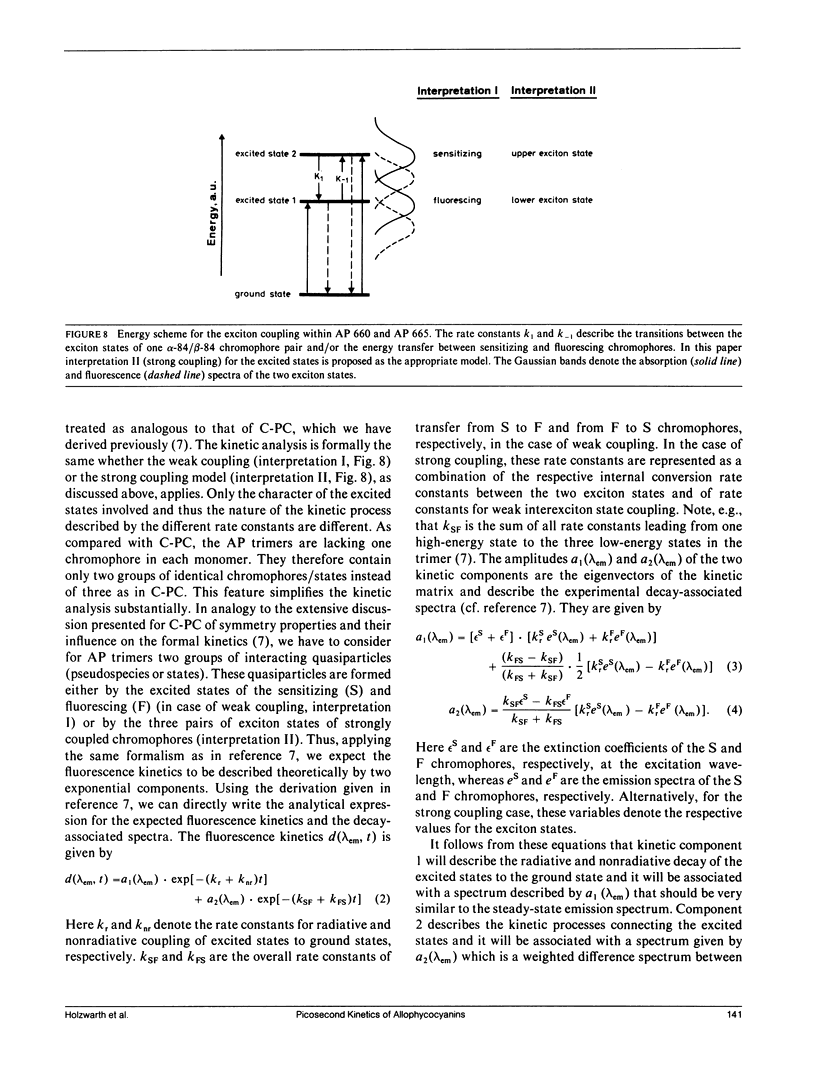
The excited state kinetics of three different allophycocyanin (AP) complexes has been studied by picosecond fluorescence spectroscopy. Both the fluorescence kinetics and the decay-associated fluorescence spectra of the different complexes can be understood on the basis of a structural model for AP which uses (a) an analogy to the known x-ray determined structure of C-phycocyanin, (b) the biochemical analogies of AP and C-phycocyanin, and (c) the biochemical composition of AP-B (AP-681). A model is developed that describes the excited state kinetics as a mixture of internal conversion processes within a coupled exciton pair and energy transfer processes between exciton pairs. We found excited state relaxation times in the range of 13 ps (AP with linker peptide) up to 66 ps (AP-B). The trimeric aggregates AP 660 and AP 665 show one fast relaxation component each, as was expected on the basis of their symmetry properties. The lower symmetry of AP-B (AP-681) gives rise to two fast lifetime components (τ1 = 23 ps and τ2 = 66 ps) which are attributed to internal conversion and/or energy transfer between excitonic states formed by the coupling of symmetrically and spectrally nonequivalent chromophores. It is proposed that the internal conversion between exciton states of strongly coupled chromophores fulfills the requirements of the small energy gap limit. Thus, internal conversion rates in the order of tens of picoseconds are feasible. The influence of the interaction of the linker peptide on the properties of the AP trimer are manifested in the fluorescence kinetics. Lack of the linker peptide in AP 660 gives rise to a heterogeneity in the chromophore conformations and chromophore-chromophore interactions.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canaani O. D., Gantt E. Circular dichroism and polarized fluorescence characteristics of blue-green algal allophycocyanins. Biochemistry. 1980 Jun 24;19(13):2950–2956. doi: 10.1021/bi00554a021. [DOI] [PubMed] [Google Scholar]
- Csatorday K., Guard-Friar D., MacColl R., Berns D. S. The development of exciton migration routes for phycocyanin 645 and allophycocyanin. Photochem Photobiol. 1988 Feb;47(2):285–291. doi: 10.1111/j.1751-1097.1988.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Csatorday K., MacColl R., Csizmadia V., Grabowski J., Bagyinka C. Exciton interaction in allophycocyanin. Biochemistry. 1984 Dec 18;23(26):6466–6470. doi: 10.1021/bi00321a029. [DOI] [PubMed] [Google Scholar]
- Csatorday K., MacColl R., Guard-Friar D., Hanzlik C. A. Excitation energy transfer between sensitizing chromophores of phycocyanin 612. Photochem Photobiol. 1987 Jun;45(6):845–848. doi: 10.1111/j.1751-1097.1987.tb07893.x. [DOI] [PubMed] [Google Scholar]
- Dagen A. J., Alfano R. R., Zilinskas B. A., Swenberg C. E. Analysis of fluorescence kinetics and energy transfer in isolated alpha subunits of phycoerythrin from Nostoc sp. Photochem Photobiol. 1986 Jan;43(1):71–79. doi: 10.1111/j.1751-1097.1986.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Mimuro M., Suter F., Zuber H. Allophycocyanin complexes of the phycobilisome from Mastigocladus laminosus. Influence of the linker polypeptide L8.9C on the spectral properties of the phycobiliprotein subunits. Biol Chem Hoppe Seyler. 1987 Apr;368(4):353–367. doi: 10.1515/bchm3.1987.368.1.353. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Comparative biochemistry of photosynthetic light-harvesting systems. Annu Rev Biochem. 1983;52:125–157. doi: 10.1146/annurev.bi.52.070183.001013. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R., Wendler J., Suter G. W. Studies on Chromophore Coupling in Isolated Phycobiliproteins: II. Picosecond Energy Transfer Kinetics and Time-Resolved Fluorescence Spectra of C-Phycocyanin from Synechococcus 6301 as a Function of the Aggregation State. Biophys J. 1987 Jan;51(1):1–12. doi: 10.1016/S0006-3495(87)83306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Song P. S., Paxton R. J., Edelstein M. S., Swanson R., Hazen E. E., Jr Molecular topography of the phycocyanin photoreceptor from Chroomonas species. Biochemistry. 1980 Jan 8;19(1):24–32. doi: 10.1021/bi00542a005. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Degenkolb E. O., Bersohn R., Rentzepis P. M., MacColl R., Berns D. S. Energy transfer among the chromophores in phycocyanins measured by picosecond kinetics. Biochemistry. 1979 Nov 13;18(23):5073–5078. doi: 10.1021/bi00590a008. [DOI] [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Allophycocyanin B. A common beta subunit in Synechococcus allophycocyanin B (lambda max 670 nm) and allophycocyanin (lambda max 650 nM). J Biol Chem. 1981 Dec 10;256(23):12600–12606. [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Core substructure in Synechococcus 6301 phycobilisomes: two new allophycocyanin and allophycocyanin B complexes. J Biol Chem. 1983 Jan 25;258(2):902–908. [PubMed] [Google Scholar]
- MacColl R., Csatorday K., Berns D. S., Traeger E. Chromophore interactions in allophycocyanin. Biochemistry. 1980 Jun 10;19(12):2817–2820. doi: 10.1021/bi00553a043. [DOI] [PubMed] [Google Scholar]
- Robinson G. W. Excitation transfer and trapping in photosynthesis. Brookhaven Symp Biol. 1966;19:16–48. [PubMed] [Google Scholar]
- Rümbeli R., Wirth M., Suter F., Zuber H. The phycobiliprotein beta 16.2 of the allophycocyanin core from the cyanobacterium Mastigocladus laminosus. Characterization and complete amino-acid sequence. Biol Chem Hoppe Seyler. 1987 Jan;368(1):1–9. doi: 10.1515/bchm3.1987.368.1.1. [DOI] [PubMed] [Google Scholar]
- Scheer J. K. Effect of placement in the order of competition on scores of Nebraska high school students. Res Q. 1973 Mar;44(1):79–85. [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Huber R., Schneider M., Bode W., Miller M., Hackert M. L. Crystal structure analysis and refinement at 2.5 A of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol Biol. 1986 Apr 20;188(4):651–676. doi: 10.1016/s0022-2836(86)80013-4. [DOI] [PubMed] [Google Scholar]
- Sidler W., Gysi J., Isker E., Zuber H. The complete amino acid sequence of both subunits of allophycocyanin, a light harvesting protein-pigment complex from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1981 Jun;362(6):611–628. doi: 10.1515/bchm2.1981.362.1.611. [DOI] [PubMed] [Google Scholar]
- Suter G. W., Holzwarth A. R. A kinetic model for the energy transfer in phycobilisomes. Biophys J. 1987 Nov;52(5):673–683. doi: 10.1016/S0006-3495(87)83262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


