Abstract
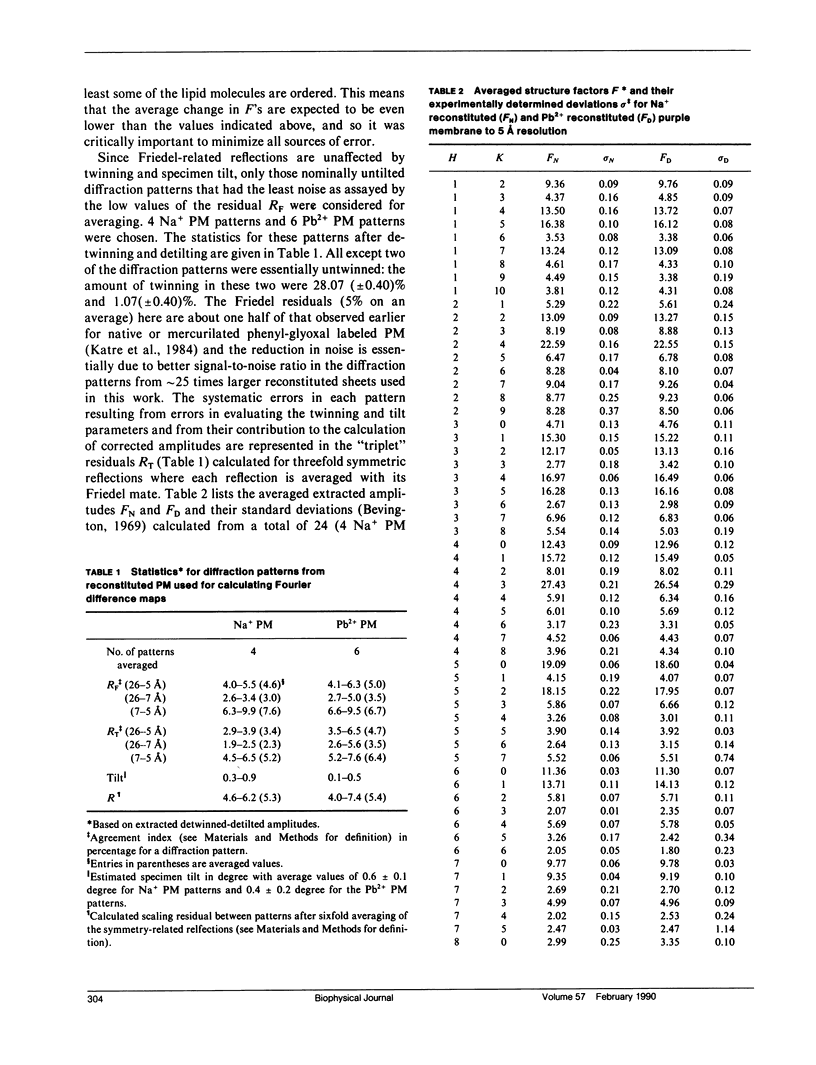
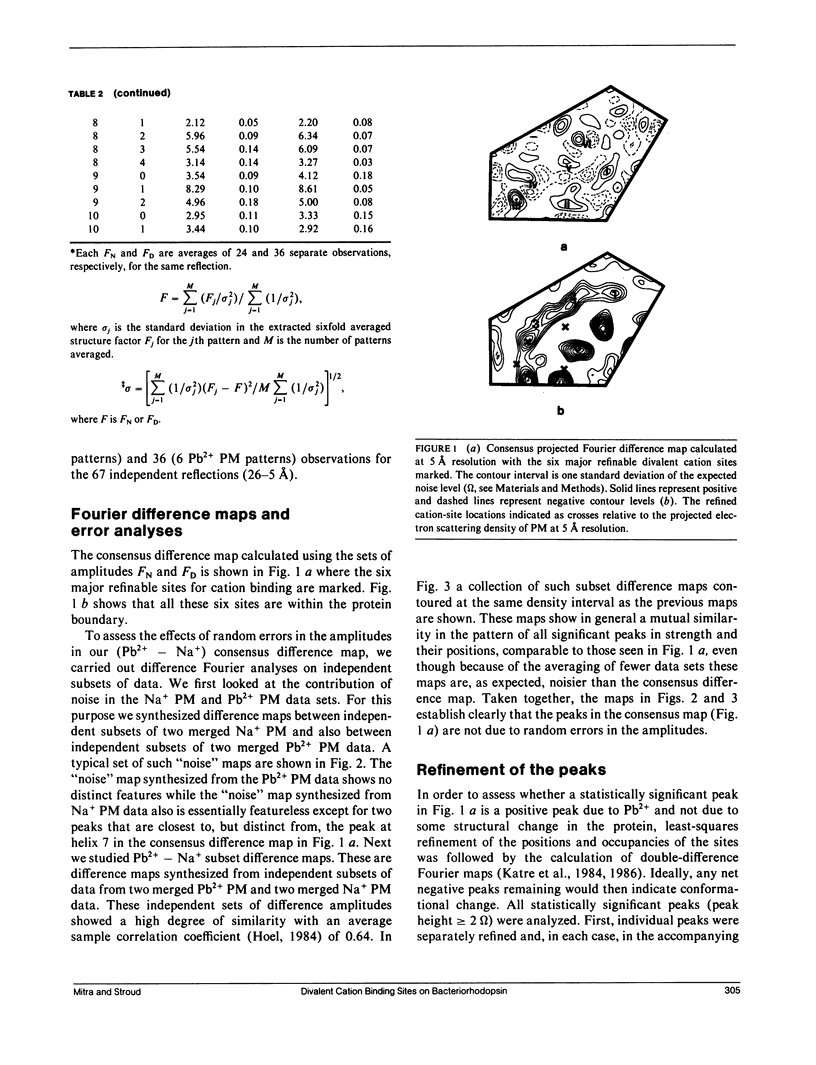
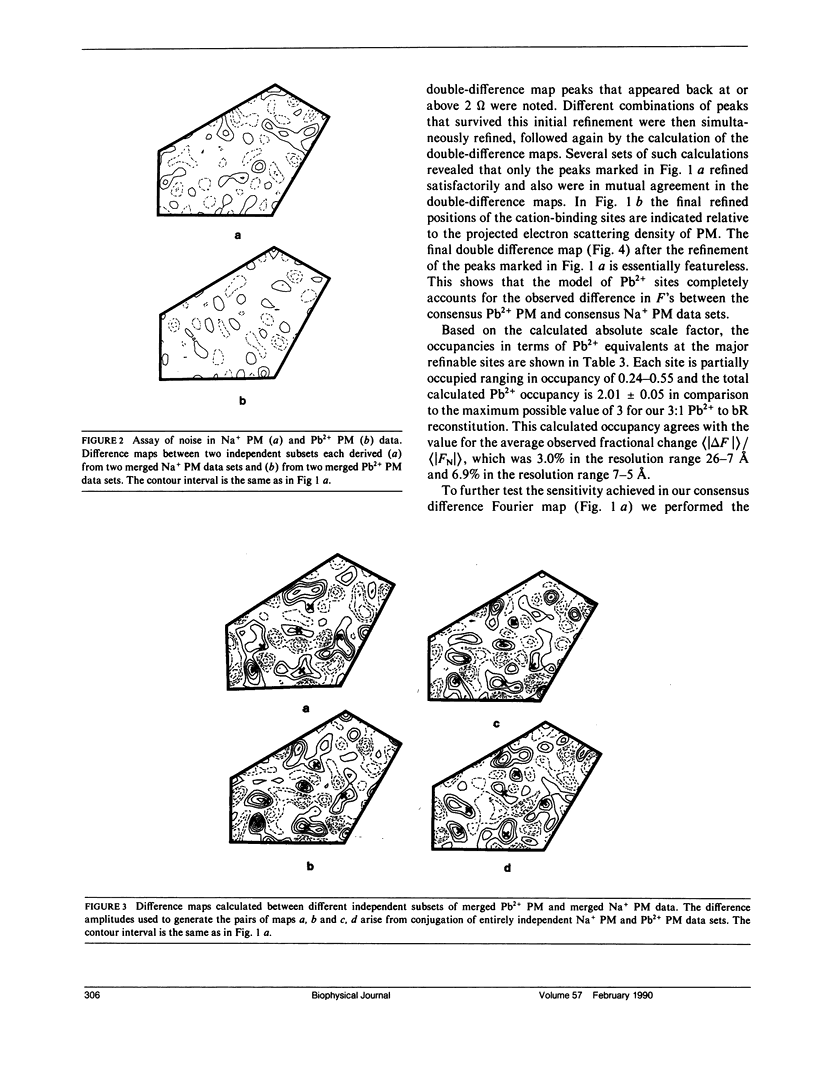
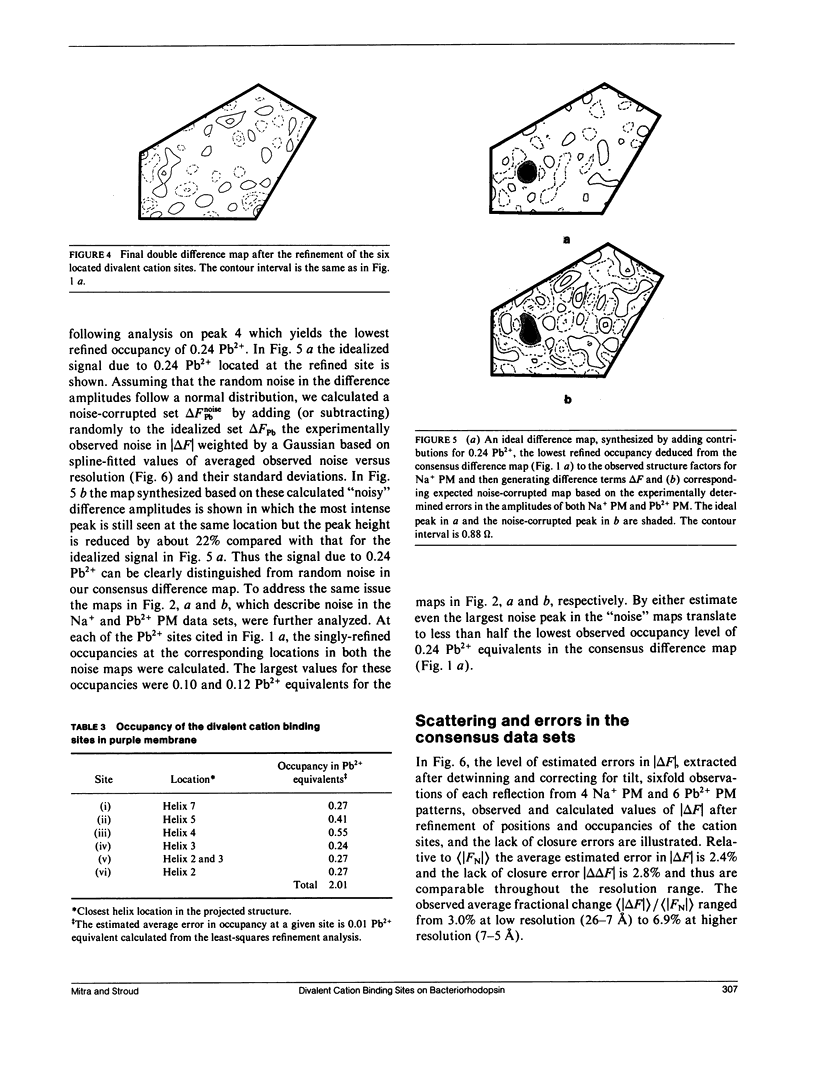
A sensitive high-resolution electron diffraction assay for change in structure is described and harnessed to analyze the binding of divalent cations to the purple membrane (PM) of Halobacterium halobium. Low-dose electron diffraction patterns are subject to a matched filter algorithm (Spencer, S. A., and A. A. Kossiakoff. 1980. J. Appl. Crystallogr. 13:563-571). to extract accurate values of reflection intensities. This, coupled with a scheme to account for twinning and specimen tilt in the microscope, yields results that are sensitive enough to rapidly quantitate any structure change in PM brought about by site-directed mutagenesis to the level of less than two carbon atoms. Removal of tightly bound divalent cations (mainly Ca2+ and Mg2+) from PM causes a color change to blue and is accompanied by a severely altered photocycle of the protein bacteriohodopsin (bR), a light-driven proton pump. We characterize the structural changes that occur upon association of 3:1 divalent cation to PM, versus membranes rendered purple by addition of excess Na+. High resolution, low dose electron diffraction data obtained from glucose-embedded samples of Pb2+ and Na+ reconstituted PM preparations at room temperature identify several sites with total occupancy of 2.01 +/- 0.05 Pb2+ equivalents. The color transition as a function of ion concentration for Ca2+ or Mg2+ and Pb2+ are strictly comparable. A (Pb2(+)-Na+) PM Fourier difference map in projection was synthesized at 5 A using the averaged data from several nominally untilted patches corrected for twinning and specimen tilt. We find six major sites located on helices 7, 5, 4, 3, 2 (nomenclature of Engelman et al. 1980. Proc. Natl. Acad. Sci. USA. 77:2023-2027) in close association with bR. These partially occupied sites (0.55-0.24 Pb2+ equivalents) represent preferential sites of binding for divalent cations and complements our earlier result by x-ray diffraction (Katre et al. 1986. Biophys. J. 50:277-284).
Full text
PDF


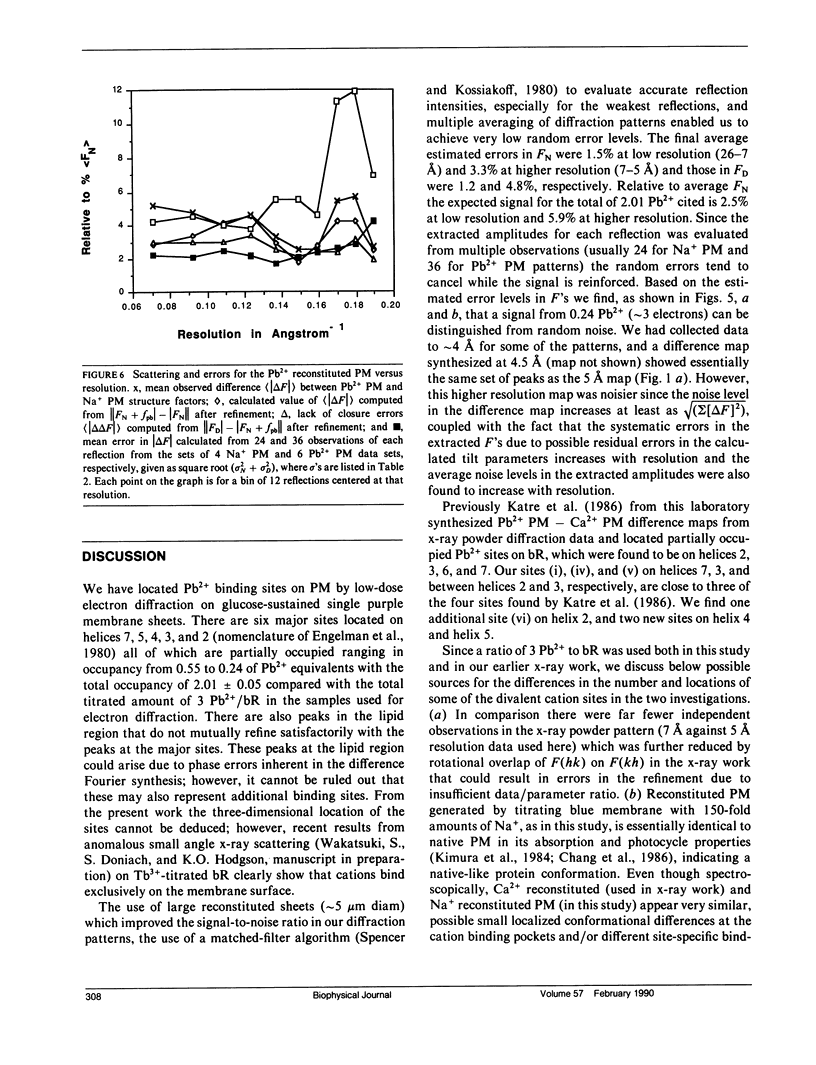



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariki M., Lanyi J. K. Characterization of metal ion-binding sites in bacteriorhodopsin. J Biol Chem. 1986 Jun 25;261(18):8167–8174. [PubMed] [Google Scholar]
- Baldwin J. M., Henderson R., Beckman E., Zemlin F. Images of purple membrane at 2.8 A resolution obtained by cryo-electron microscopy. J Mol Biol. 1988 Aug 5;202(3):585–591. doi: 10.1016/0022-2836(88)90288-4. [DOI] [PubMed] [Google Scholar]
- Bayley H., Huang K. S., Radhakrishnan R., Ross A. H., Takagaki Y., Khorana H. G. Site of attachment of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2225–2229. doi: 10.1073/pnas.78.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Light-driven proton translocations in Halobacterium halobium. Biochim Biophys Acta. 1976 Jul 9;440(1):68–88. doi: 10.1016/0005-2728(76)90114-6. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Jonas R., Melchiore S., Govindjee R., Ebrey T. G. Mechanism and role of divalent cation binding of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):731–739. doi: 10.1016/S0006-3495(86)83699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Müller U. Temperature-dependent aggregation of bacteriorhodopsin in dipalmitoyl- and dimyristoylphosphatidylcholine vesicles. J Mol Biol. 1978 May 15;121(2):283–298. doi: 10.1016/s0022-2836(78)80010-2. [DOI] [PubMed] [Google Scholar]
- Corcoran T. C., Ismail K. Z., El-Sayed M. A. Evidence for the involvement of more than one metal cation in the Schiff base deprotonation process during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4094–4098. doi: 10.1073/pnas.84.12.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis P., Corcoran T. C., El-Sayed M. A. Importance of bound divalent cations to the tyrosine deprotonation during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3662–3664. doi: 10.1073/pnas.82.11.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duñach M., Padrós E., Seigneuret M., Rigaud J. L. On the molecular mechanism of the blue to purple transition of bacteriorhodopsin. UV-difference spectroscopy and electron spin resonance studies. J Biol Chem. 1988 Jun 5;263(16):7555–7559. [PubMed] [Google Scholar]
- Duñach M., Seigneuret M., Rigaud J. L., Padrós E. The relationship between the chromophore moiety and the cation binding sites in bacteriorhodopsin. Biosci Rep. 1986 Nov;6(11):961–966. doi: 10.1007/BF01114972. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jubb J. S., Henderson R. Structural comparison of native and deoxycholate-treated purple membrane. Biophys J. 1985 Nov;48(5):775–780. doi: 10.1016/S0006-3495(85)83835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Jubb J. S., Whytock S. Specific labelling of the protein and lipid on the extracellular surface of purple membrane. J Mol Biol. 1978 Aug 5;123(2):259–274. doi: 10.1016/0022-2836(78)90325-x. [DOI] [PubMed] [Google Scholar]
- Jaffe J. S., Glaeser R. M. Difference Fourier analysis of "surface features" of bacteriorhodopsin using glucose-embedded and frozen-hydrated purple membrane. Ultramicroscopy. 1987;23(1):17–28. doi: 10.1016/0304-3991(87)90223-3. [DOI] [PubMed] [Google Scholar]
- Katre N. V., Finer-Moore J., Stroud R. M., Hayward S. B. Location of an extrinsic label in the primary and tertiary structure of bacteriorhodopsin. Biophys J. 1984 Aug;46(2):195–203. doi: 10.1016/S0006-3495(84)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Kimura Y., Stroud R. M. Cation binding sites on the projected structure of bacteriorhodopsin. Biophys J. 1986 Aug;50(2):277–284. doi: 10.1016/S0006-3495(86)83461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Wolber P. K., Stoeckenius W., Stroud R. M. Attachment site(s) of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4068–4072. doi: 10.1073/pnas.78.7.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Packer L., Arrio B., Johannin G., Volfin P. Surface charge of purple membranes measured by laser Doppler velocimetry. Biochem Biophys Res Commun. 1984 Jul 18;122(1):252–258. doi: 10.1016/0006-291x(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Effect of lipid surface charges on the purple-to-blue transition of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3681–3684. doi: 10.1073/pnas.84.11.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Purple-to-blue transition of bacteriorhodopsin in a neutral lipid environment. Biophys J. 1988 Aug;54(2):227–232. doi: 10.1016/S0006-3495(88)82951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Surface pH controls purple-to-blue transition of bacteriorhodopsin. A theoretical model of purple membrane surface. Biophys J. 1989 Aug;56(2):369–383. doi: 10.1016/S0006-3495(89)82683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


