Abstract
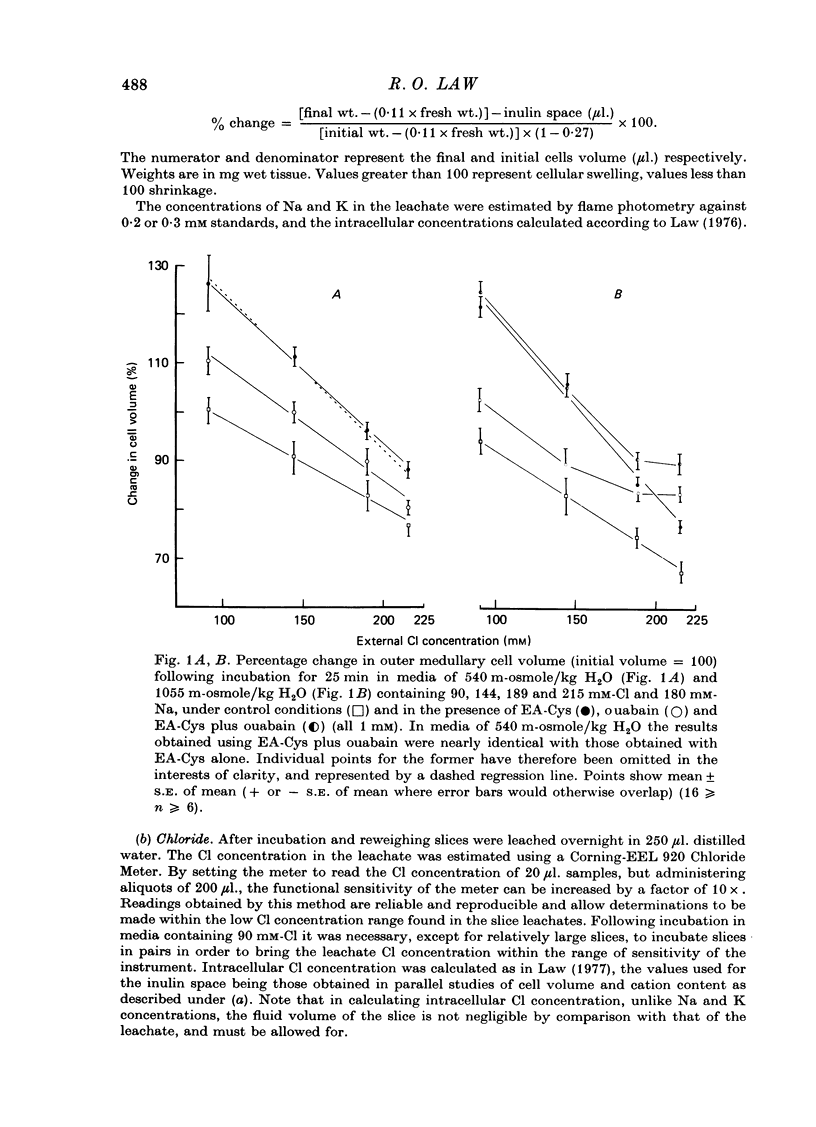
1. Slices of rat renal outer medulla have been incubated in media made hyperosmotic (540 and 1055 m-osmole/kg H2O) by the addition of urea, and containing variable concentrations of Cl (90, 144, 189 and 215 mM) and constant concentrations of Na (180 mM) and K (5.9 mM). A small number of incubations have been conducted in the presence of 100 mM-Na. 2. Changes in cell volume during incubation have been calculated on the basis of initial and final slice weight and inulin space. 3. The capacity of cells to shrink in response to extracellular osmotic stress was related principally to the external Cl concentration rather than to osmolality, increases in concentration being associated with enhanced shrinkage. Shrinkage was accompanied by net loss of cellular Cl. The ratio between intra- and extracellular Cl concentration (ca. 0.41) remained constant in all media. 4. In media containing low Cl concentration (90 mM), reduction of media Na concentration to 100 mM enhanced shrinkage. This effect was not observed when medium Cl concentration was greater than 90 mM. 5. Ethacrynic acid-cysteine (1 mM) significantly impaired the shrinkage response to extracellular osmotic stress, and caused cell swelling in media of relatively low Cl concentration and osmolality. It did not abolish the dependency of cell volume upon Cl concentration. There was marked reduction in the net amount of Cl lost from cells. 6. Ethacrynic acid-cysteine caused an increase in cellular Na content only in media containing 540 m-osmole/kg H2O and Cl concentrations less than 215 mM. 7. Ouabain (1 mM) inhibited cell shrinkage to a lesser extent than ethacrynic acid-cysteine in all media except that causing the greatest shrinkage under control conditions (215 mM-Cl/1055 m-osmole/kg H2O). It is suggested that a ouabain-sensitive process may play an increasingly important role in Cl-related cell shrinkage as this becomes more pronounced. 8. The findings are consistent with the view that Cl ions influence cell volume both through their effective external osmotic pressure and by means of Cl-specific process; the latter is associated with net loss of cellular Cl. A dependence of this loss upon Na/k exchange-linked metabolism is inferred, but the present findings do not permit the active or passive nature of the extrusion to be defined.
Full text
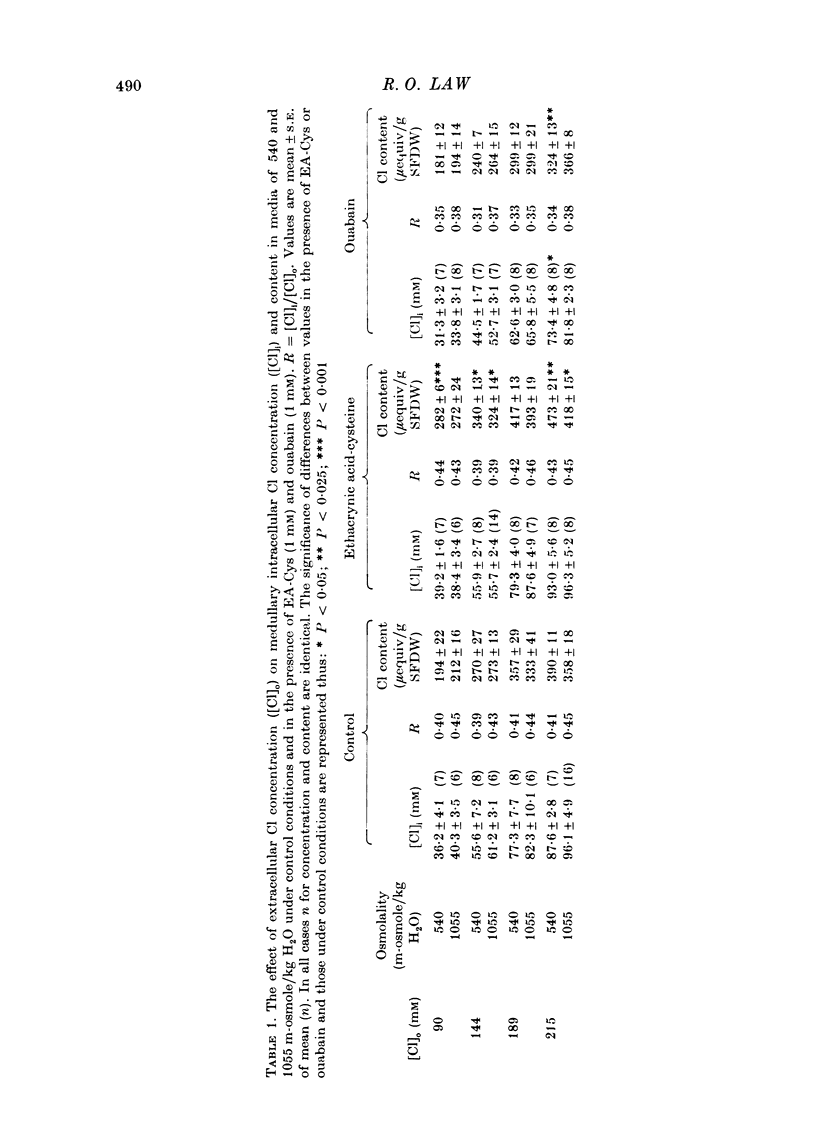
PDF



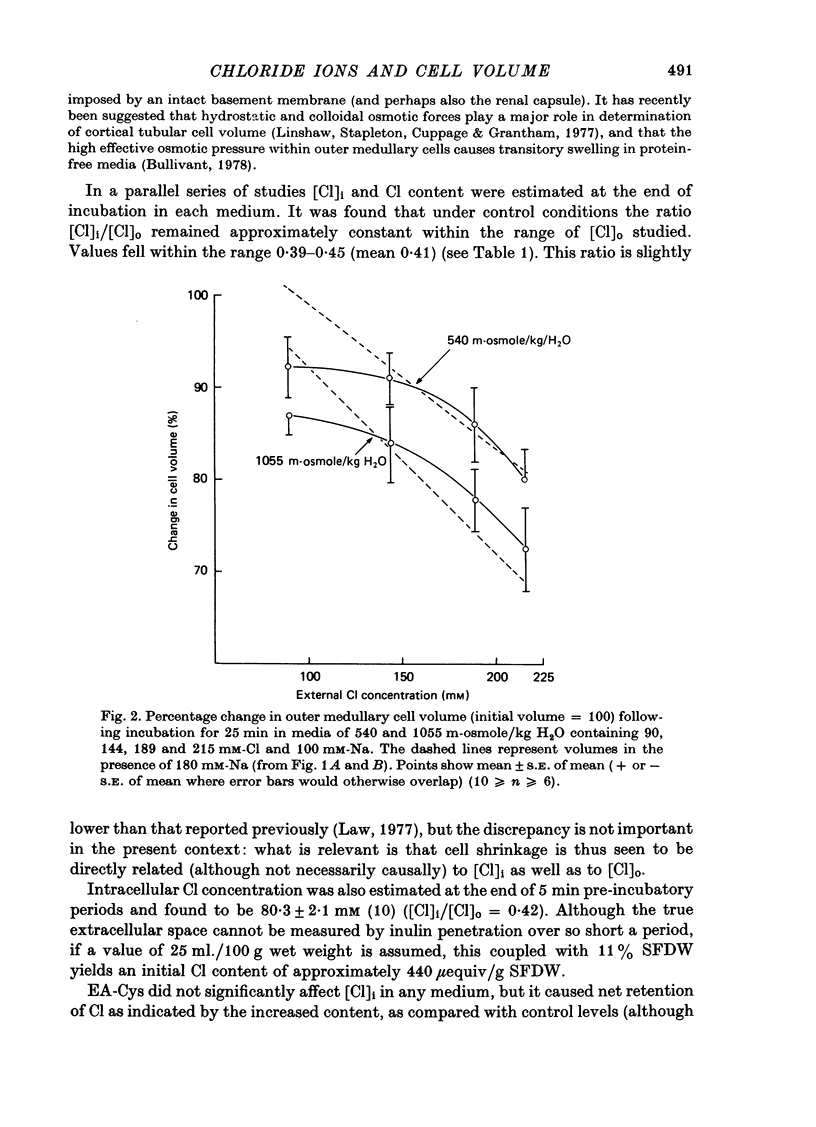
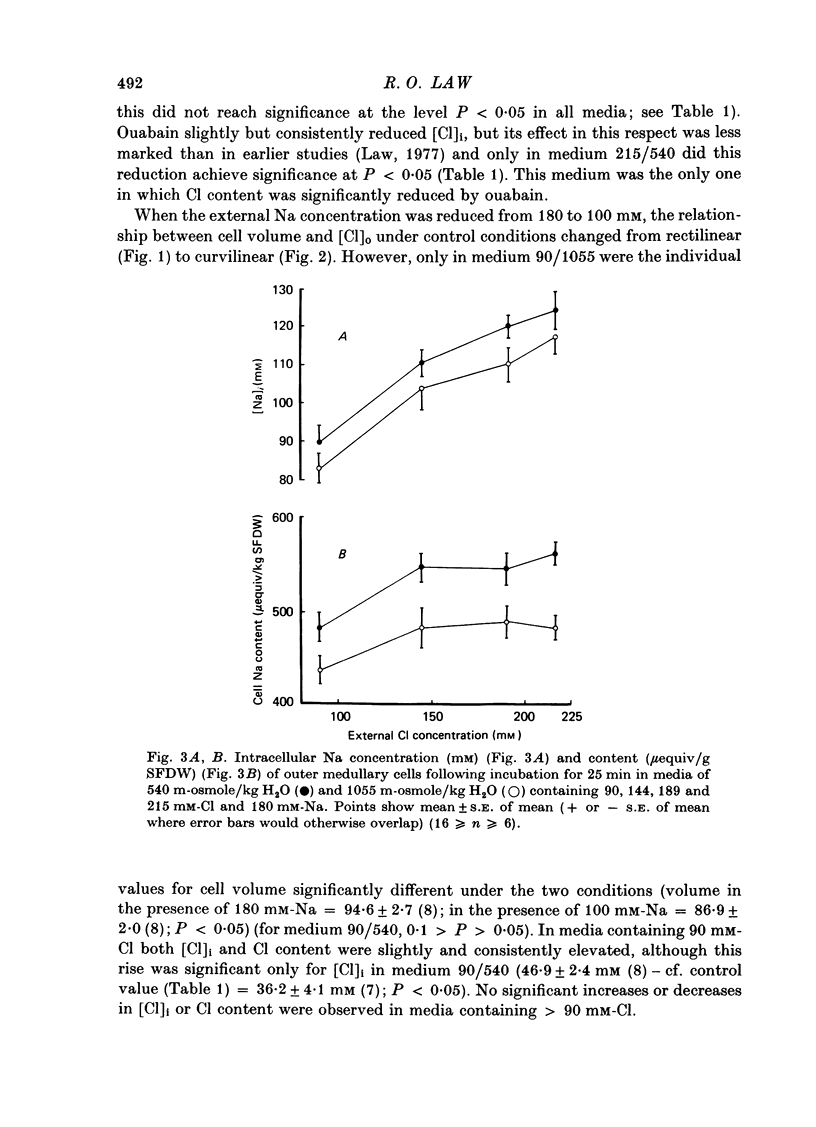
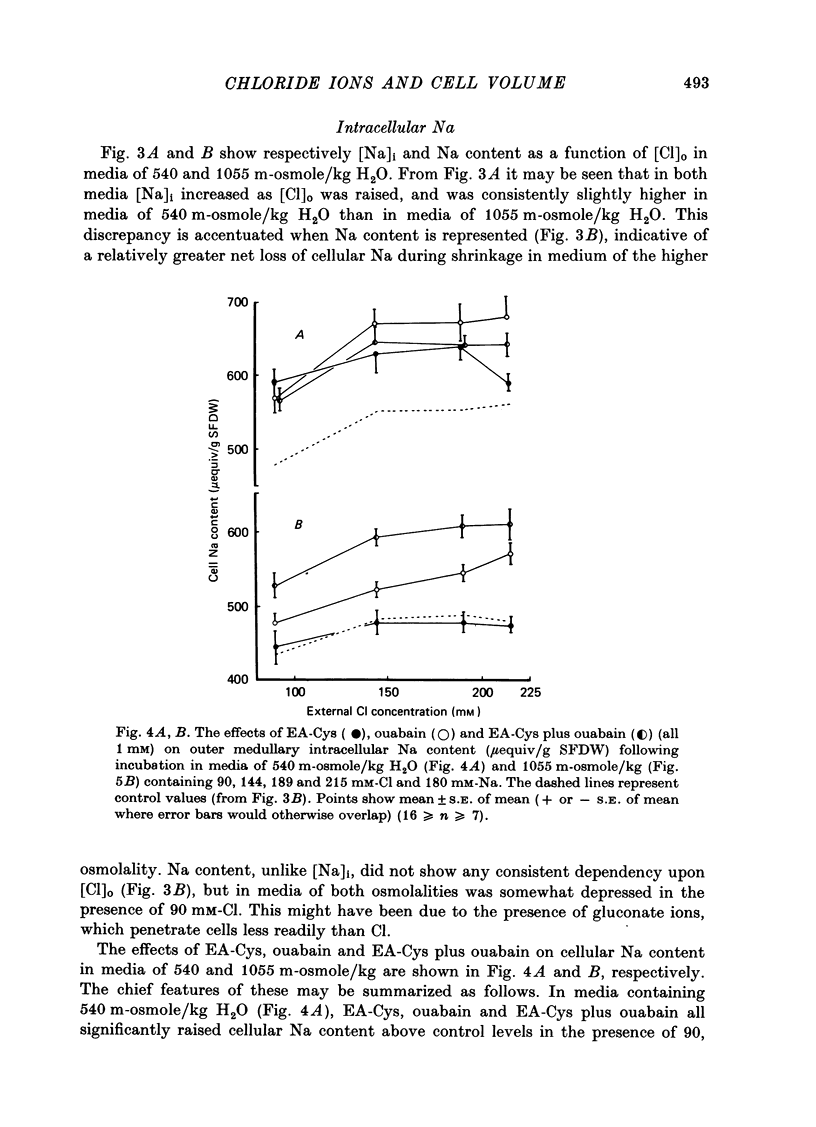

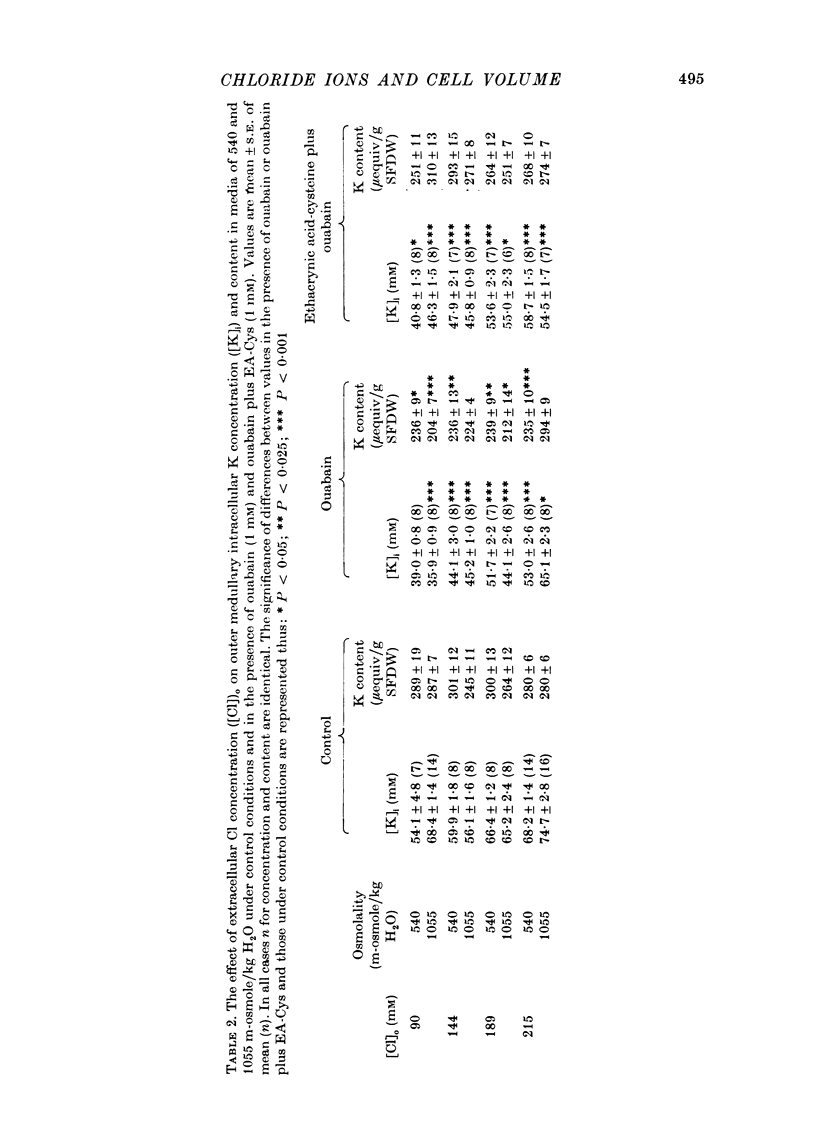

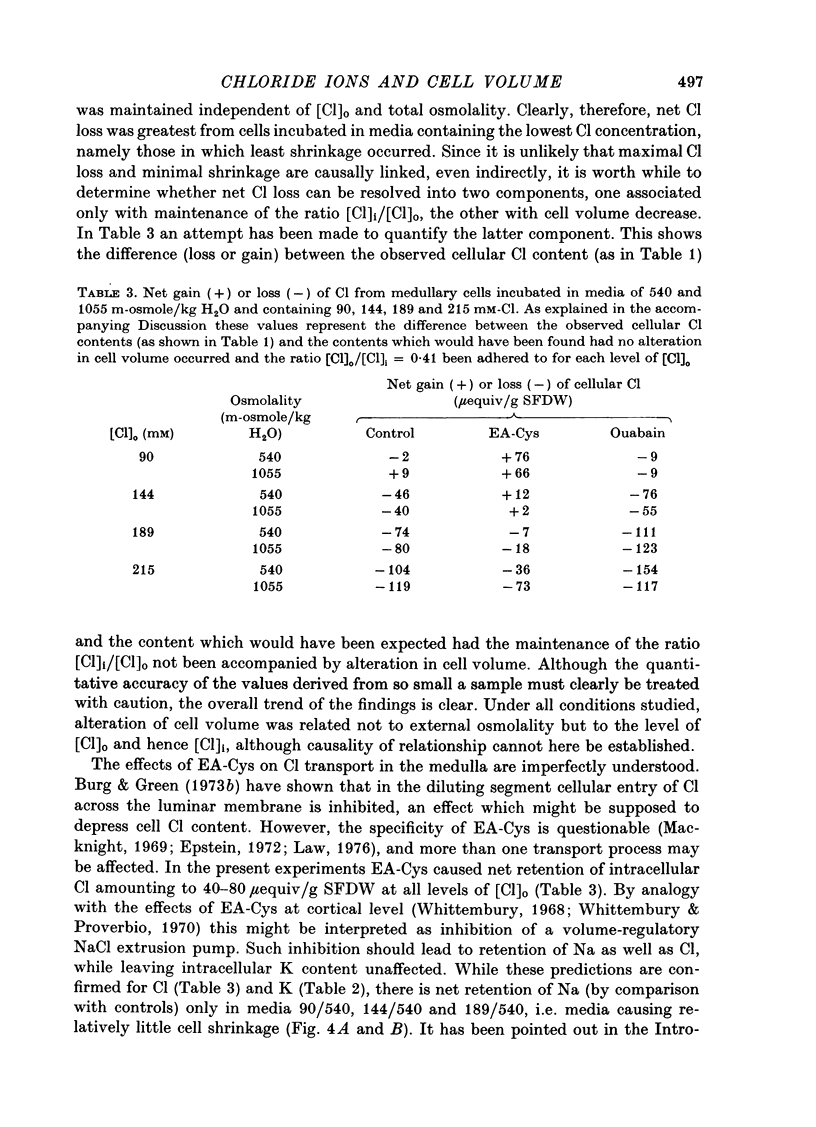



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. C., Lee J. B. Effect of osmolality on Na plus-K plus-ATPase in outer renal medulla. Am J Physiol. 1970 Dec;219(6):1742–1745. doi: 10.1152/ajplegacy.1970.219.6.1742. [DOI] [PubMed] [Google Scholar]
- Bullivant M. Volume changes in cells of the outer medulla during perfusion of the rat kidney. J Physiol. 1978 Jul;280:125–139. doi: 10.1113/jphysiol.1978.sp012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M., Green N. Effect of ethacrynic acid on the thick ascending limb of Henle's loop. Kidney Int. 1973 Nov;4(5):301–308. doi: 10.1038/ki.1973.121. [DOI] [PubMed] [Google Scholar]
- Dellasega M., Grantham J. J. Regulation of renal tubule cell volume in hypotonic media. Am J Physiol. 1973 Jun;224(6):1288–1294. doi: 10.1152/ajplegacy.1973.224.6.1288. [DOI] [PubMed] [Google Scholar]
- Epstein R. W. The effects of ethacrynic acid on active transport of sugars and ions and on other metabolic processes in rabbit kidney cortex. Biochim Biophys Acta. 1972 Jul 3;274(1):128–139. doi: 10.1016/0005-2736(72)90288-x. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Boulpaep E. L., Whittembury G. Electrolyte transport in kidney tubule cells. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):175–196. doi: 10.1098/rstb.1971.0088. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Gross J. B., Kawamura S., Waters J. D., Kokko J. P. Electrophysiological study of isolated perfused human collecting ducts: Ion dependency of the transepithelial potential difference. J Clin Invest. 1976 Nov;58(5):1233–1239. doi: 10.1172/JCI108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinzeller A., Nedvídková J., Knotková A. Effect of saline osmolarity on the steady-state level of water and electrolytes in kidney cortex cells. Biochim Biophys Acta. 1967 May 2;135(2):286–299. doi: 10.1016/0005-2736(67)90122-8. [DOI] [PubMed] [Google Scholar]
- Knox W. H., Sax J. A., Wilson D. R., Sen A. K. Effect of osmolality on renal medullary Na-K-ATPase activity in the postobstructive kidney. Can J Physiol Pharmacol. 1977 Oct;55(5):1112–1115. doi: 10.1139/y77-153. [DOI] [PubMed] [Google Scholar]
- Law R. O. Factors affecting the distribution of chloride ions in rat renal outer medulla. J Physiol. 1977 Mar;266(1):173–189. doi: 10.1113/jphysiol.1977.sp011762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. The effects of ouabain and ethacrynic acid on the intracellular sodium and potassium concentrations in renal medullary slices incubated in cold potassium-free ringer solution and re-incubated at 37 degrees C in the presence of external potassium. J Physiol. 1976 Jan;254(3):743–758. doi: 10.1113/jphysiol.1976.sp011256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. The influence of external chloride concentration on the volume of renal medullary cells in hyperosmolal media [proceedings]. J Physiol. 1978 Feb;275:26P–27P. [PubMed] [Google Scholar]
- Law R. O. The inulin space, solute concentrations, and weight changes in rat renal medullary slices incubated in iso-osmolal media, and their modification during anoxia and hypothermia. J Physiol. 1975 May;247(1):37–54. doi: 10.1113/jphysiol.1975.sp010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linshaw M. A., Stapleton F. B., Cuppage F. E., Grantham J. J. Effect of basement membrane and colloid osmotic pressure on renal tubule cell volume. Am J Physiol. 1977 Oct;233(4):F325–F332. doi: 10.1152/ajprenal.1977.233.4.F325. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. The effects of ethacrynic acid on the electrolyte and water contents of rat renal cortical slices. Biochim Biophys Acta. 1969 Mar 11;173(2):223–233. doi: 10.1016/0005-2736(69)90106-0. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Guiñazú A., Arrizurieta E. E., Yelinek L. Electrolyte, water, and urea content in dog kidneys in different states of diuresis. Am J Physiol. 1964 Apr;206(4):725–730. doi: 10.1152/ajplegacy.1964.206.4.725. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Grantham J. J. Cellular aspects of renal sodium transport and cell volume regulation. Kidney Int. 1976 Feb;9(2):103–120. doi: 10.1038/ki.1976.15. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]
- Williams M. M., Moffat D. B., Creasey M. The effect of antidiuretic hormone on the permeability of the vessels of the renal medulla of the rat during water diuresis. Q J Exp Physiol Cogn Med Sci. 1971 Oct;56(4):250–256. doi: 10.1113/expphysiol.1971.sp002126. [DOI] [PubMed] [Google Scholar]


