Abstract
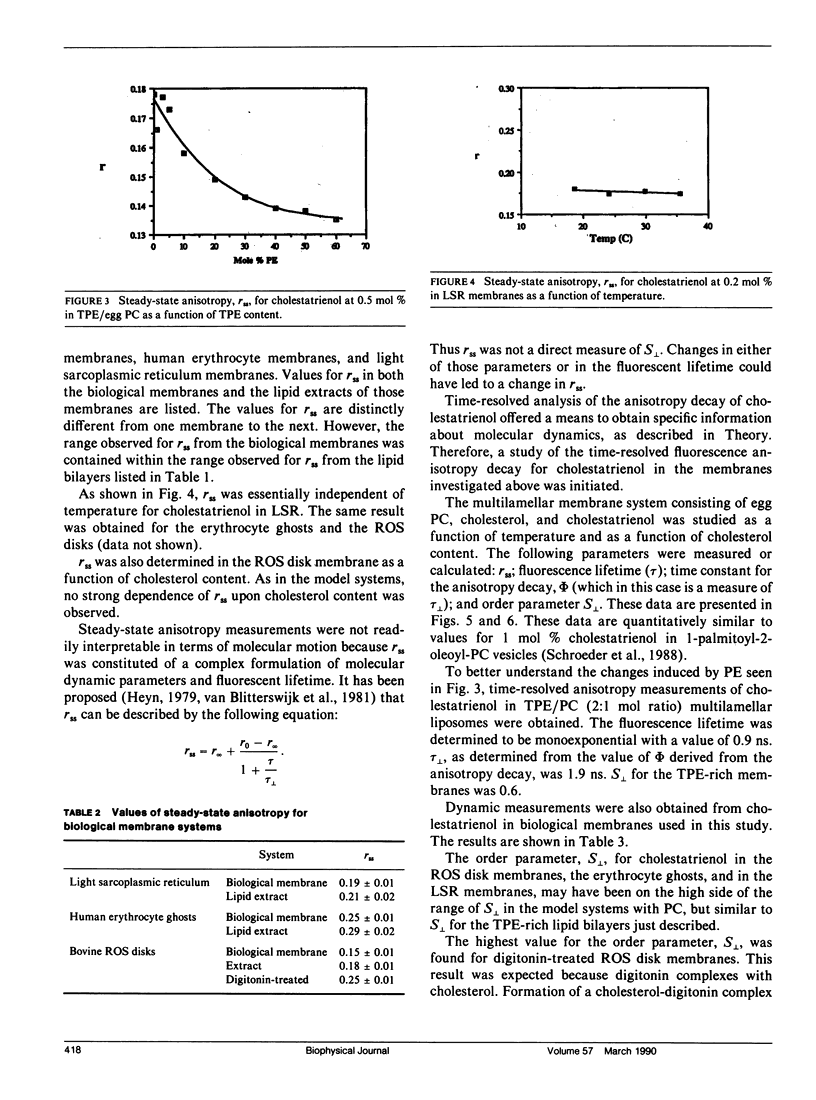
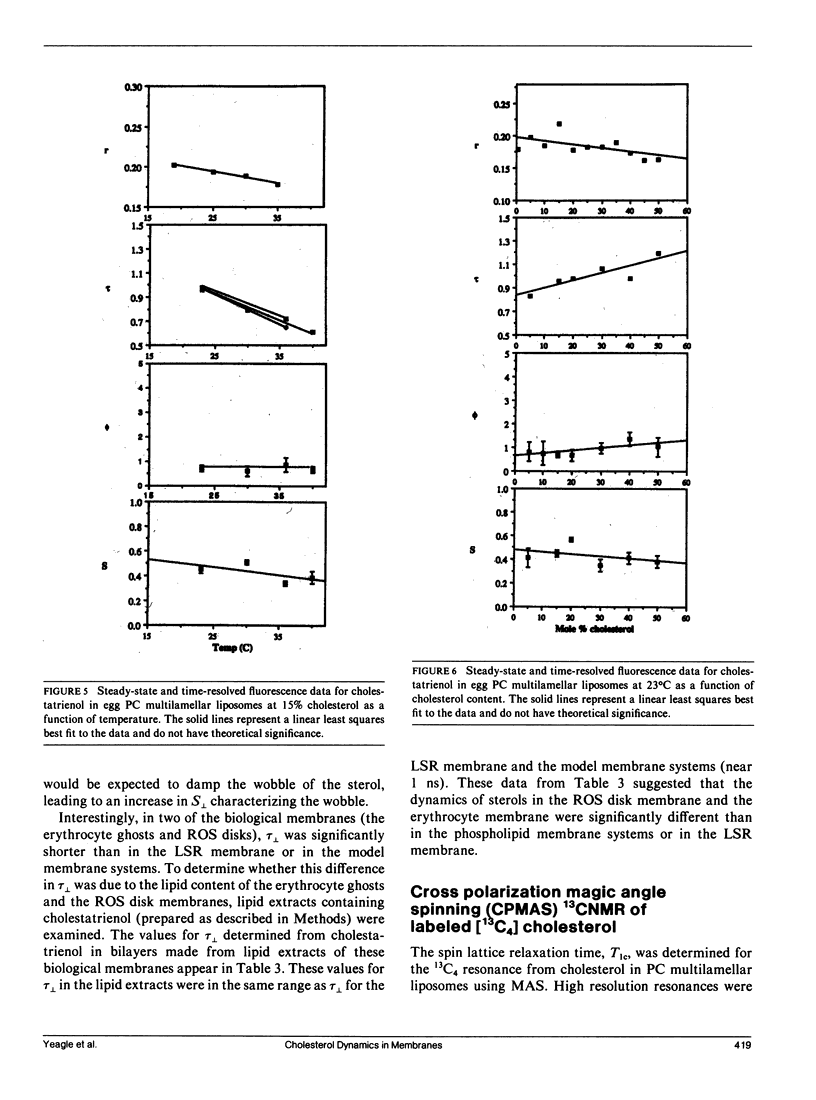
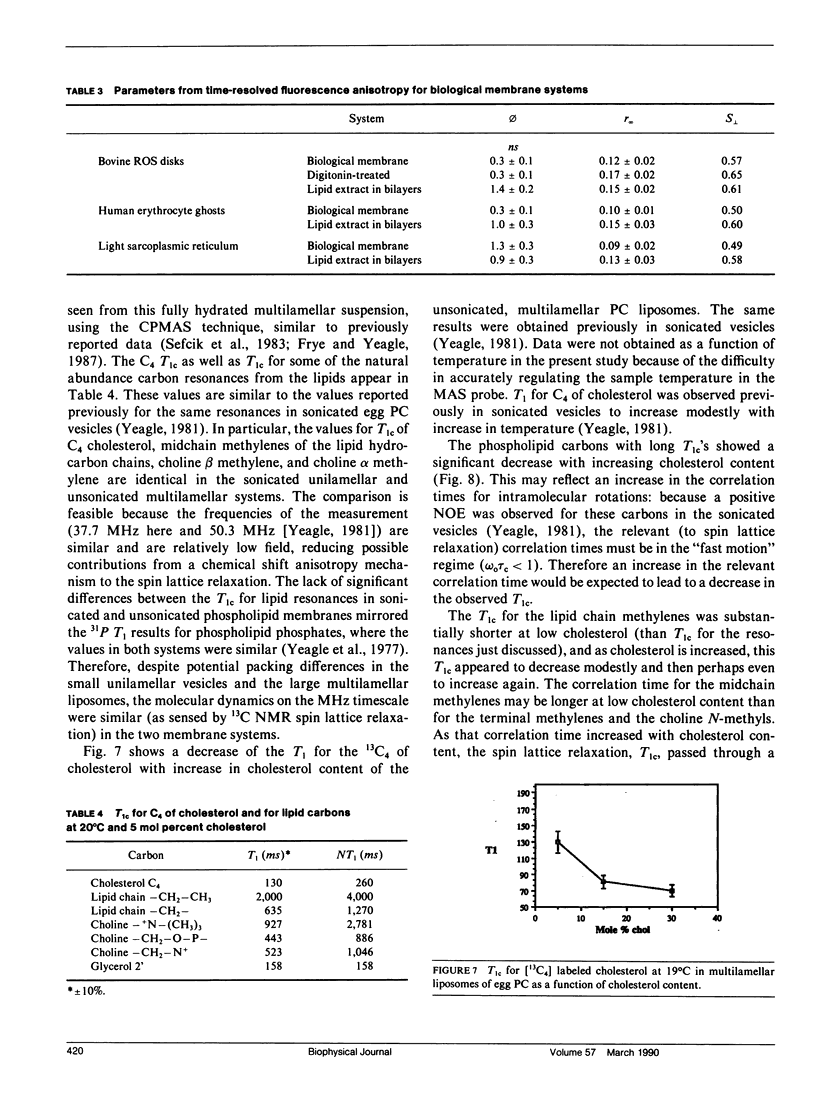
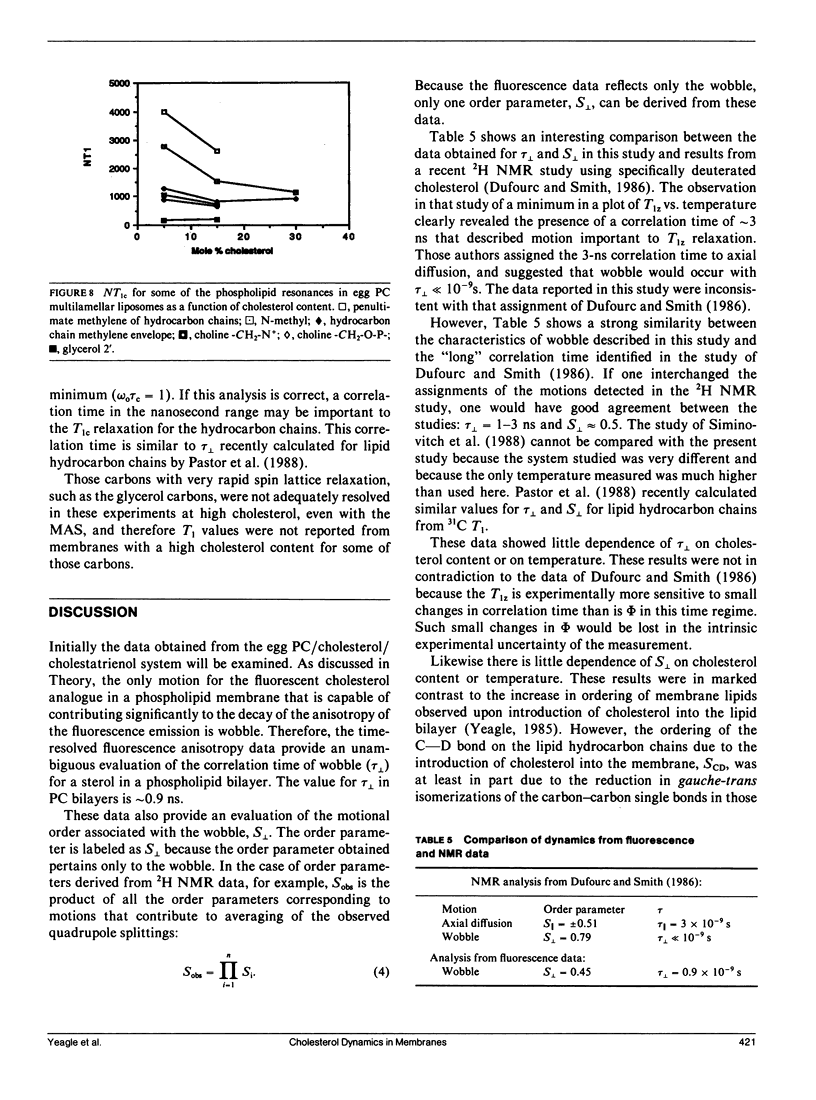
Time-resolved fluorescence anisotropy of the sterol analogue, cholestatrienol, and 13C nuclear magnetic resonance (NMR) spin lattice relaxation time (T1c) measurements of [13C4] labeled cholesterol were exploited to determine the correlation times characterizing the major modes of motion of cholesterol in unsonicated phospholipid multilamellar liposomes. Two modes of motion were found to be important: (a) rotational diffusion and (b) time dependence of the orientation of the director for axial diffusion, or "wobble." From the time-resolved fluorescence anisotropy decays of cholestatrienol in egg phosphatidylcholine (PC) bilayers, a value for tau perpendicular, the correlation time for wobble, of 0.9 x 10(-9) s and a value for S perpendicular, the order parameter characterizing the same motion, of 0.45 s were calculated. Both tau perpendicular and S perpendicular were relatively insensitive to temperature and cholesterol content of the membranes. The T1c measurements of [13C4] labeled cholesterol did not provide a quantitative determination of tau parallel, the correlation time for axial diffusion. T1c from the lipid hydrocarbon chains suggested a value for tau perpendicular similar to that for cholesterol. Steady-state anisotropy measurements and time-resolved anisotropy measurements of cholestatrienol were used to probe sterol behavior in a variety of pure and mixed lipid multilamellar liposomes. Both the lipid headgroups and the lipid hydrocarbons chains contributed to the determination of the sterol environment in the membrane, as revealed by these fluorescence measurements. In particular, effects of the phosphatidylethanolamine (PE) headgroup and of multiple unsaturation in the lipid hydrocarbon chains were observed. However, while the steady-state anisotropy was sensitive to these factors, the time-resolved fluorescence analysis indicated that tau perpendicular was not strongly affected by the lipid composition of the membrane. S perpendicular may be increased by the presence of PE. Both steady-state anisotropy measurements and time-resolved anisotropy measurements of cholestatrienol were used to probe sterol behavior in three biological membranes: bovine rod outer segment (ROS) disk membranes, human erythrocyte plasma membranes, and light rabbit muscle sarcoplasmic reticulum membranes. In the ROS disk membranes the value for S perpendicular was marginally higher than in the PC membranes, perhaps reflecting the influence of PE. The dramatic difference noted was in the value for tau perpendicular. In both the ROS disk membranes and the erythrocyte membranes, tau perpendicular was one-third to one-fifth of tau perpendicular in the phospholipid bilayers. This result may reveal an influence of membrane proteins on sterol behavior.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A. D., Sen A., Yeagle P. L. The effect of calcium on the bilayer stability of lipids from bovine rod outer segment disk membranes. Biochim Biophys Acta. 1984 Mar 28;771(1):28–34. doi: 10.1016/0005-2736(84)90106-8. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K., Hennessey T., Albert A. D. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J Biol Chem. 1989 May 15;264(14):8151–8155. [PMC free article] [PubMed] [Google Scholar]
- Brainard J. R., Szabo A. Theory for nuclear magnetic relaxation of probes in anisotropic systems: application of cholesterol in phospholipid vesicles. Biochemistry. 1981 Aug 4;20(16):4618–4628. doi: 10.1021/bi00519a016. [DOI] [PubMed] [Google Scholar]
- Bridges C. D. A method for preparing stable digitonin solutions for visual pigment extraction. Vision Res. 1977 Feb;17(2):301–302. doi: 10.1016/0042-6989(77)90095-5. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Smith I. C. A detailed analysis of the motions of cholesterol in biological membranes by 2H-NMR relaxation. Chem Phys Lipids. 1986 Sep;41(2):123–135. doi: 10.1016/0009-3084(86)90004-6. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Schroeder F., Dempsey M. E., Fischer R. T. Sterol and squalene carrier protein interactions with fluorescent delta 5,7,9(11)-cholestatrien-3 beta-ol. J Biol Chem. 1985 Mar 10;260(5):2904–2911. [PubMed] [Google Scholar]
- Schroeder F., Nemecz G., Gratton E., Barenholz Y., Thompson T. E. Fluorescence properties of cholestatrienol in phosphatidylcholine bilayer vesicles. Biophys Chem. 1988 Oct;32(1):57–72. doi: 10.1016/0301-4622(88)85034-8. [DOI] [PubMed] [Google Scholar]
- Sefcik M. D., Schaefer J., Stejskal E. O., McKay R. A., Ellena J. F., Dodd S. W., Brown M. F. Lipid bilayer dynamics and rhodopsin-lipid interactions: new approach using high-resolution solid-state 13C NMR. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1048–1055. doi: 10.1016/0006-291x(83)90668-x. [DOI] [PubMed] [Google Scholar]
- Selinsky B. S., Yeagle P. L. Two populations of phospholipids exist in sarcoplasmic reticulum and in recombined membranes containing Ca-ATPase. Biochemistry. 1984 May 8;23(10):2281–2288. doi: 10.1021/bi00305a030. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Ruocco M. J., Olejniczak E. T., Das Gupta S. K., Griffin R. G. Anisotropic 2H-nuclear magnetic resonance spin-lattice relaxation in cerebroside- and phospholipid-cholesterol bilayer membranes. Biophys J. 1988 Sep;54(3):373–381. doi: 10.1016/S0006-3495(88)82970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975 Mar;20(3):211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- Smutzer G., Crawford B. F., Yeagle P. L. Physical properties of the fluorescent sterol probe dehydroergosterol. Biochim Biophys Acta. 1986 Nov 17;862(2):361–371. doi: 10.1016/0005-2736(86)90239-7. [DOI] [PubMed] [Google Scholar]
- Smutzer G., Yeagle P. L. A fluorescence anisotropy study on the phase behavior of dimyristoylphosphatidylcholine/cholesterol mixtures. Biochim Biophys Acta. 1985 Apr 11;814(2):274–280. doi: 10.1016/0005-2736(85)90445-6. [DOI] [PubMed] [Google Scholar]
- Stone W. L., Farnsworth C. C., Dratz E. A. A reinvestigation of the fatty acid content of bovine, rat and frog retinal rod outer segments. Exp Eye Res. 1979 Apr;28(4):387–397. doi: 10.1016/0014-4835(79)90114-3. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Van Hoeven R. P., Van der Meer B. W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981 Jun 22;644(2):323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Houslay M. D., Metcalfe J. C., Birdsall N. J. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975 Jun 26;255(5511):684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol modulation of (Na+ + K+)-ATPase ATP hydrolyzing activity in the human erythrocyte. Biochim Biophys Acta. 1983 Jan 5;727(1):39–44. doi: 10.1016/0005-2736(83)90366-8. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol rotation in phospholipid vesicles as observed by 13C-NMR. Biochim Biophys Acta. 1981 Jan 8;640(1):263–273. doi: 10.1016/0005-2736(81)90551-4. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Frye J. Effects of unsaturation on 2H-NMR quadrupole splittings and 13C-NMR relaxation in phospholipid bilayers. Biochim Biophys Acta. 1987 May 29;899(2):137–142. doi: 10.1016/0005-2736(87)90393-2. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Martin R. B. Molecular dynamics of the local anesthetic tetracaine in phospholipid vesicles. Biochim Biophys Acta. 1977 Mar 1;465(2):173–178. doi: 10.1016/0005-2736(77)90071-2. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Incorporation of the human erythrocyte sialoglycoprotein into recombined membranes containing cholesterol. J Membr Biol. 1984;78(3):201–210. doi: 10.1007/BF01925968. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Young J., Rice D. Effects of cholesterol on (Na+,K+)-ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry. 1988 Aug 23;27(17):6449–6452. doi: 10.1021/bi00417a037. [DOI] [PubMed] [Google Scholar]


